
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Search
- Page Path
- HOME > Search
- SGLT2 Inhibitors and GLP-1 Agonists: A Beacon of Hope for Stroke Prevention in Diabetes
- Jae-Han Jeon
- Diabetes Metab J. 2024;48(2):213-214. Published online March 22, 2024
- DOI: https://doi.org/10.4093/dmj.2024.0079
- 862 View
- 128 Download
- Basic research
- Reducing Oxidative Stress and Inflammation by Pyruvate Dehydrogenase Kinase 4 Inhibition Is Important in Prevention of Renal Ischemia-Reperfusion Injury in Diabetic Mice
- Ah Reum Khang, Dong Hun Kim, Min-Ji Kim, Chang Joo Oh, Jae-Han Jeon, Sung Hee Choi, In-Kyu Lee
- Received June 22, 2023 Accepted July 13, 2023 Published online February 1, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0196 [Epub ahead of print]
- 773 View
- 75 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
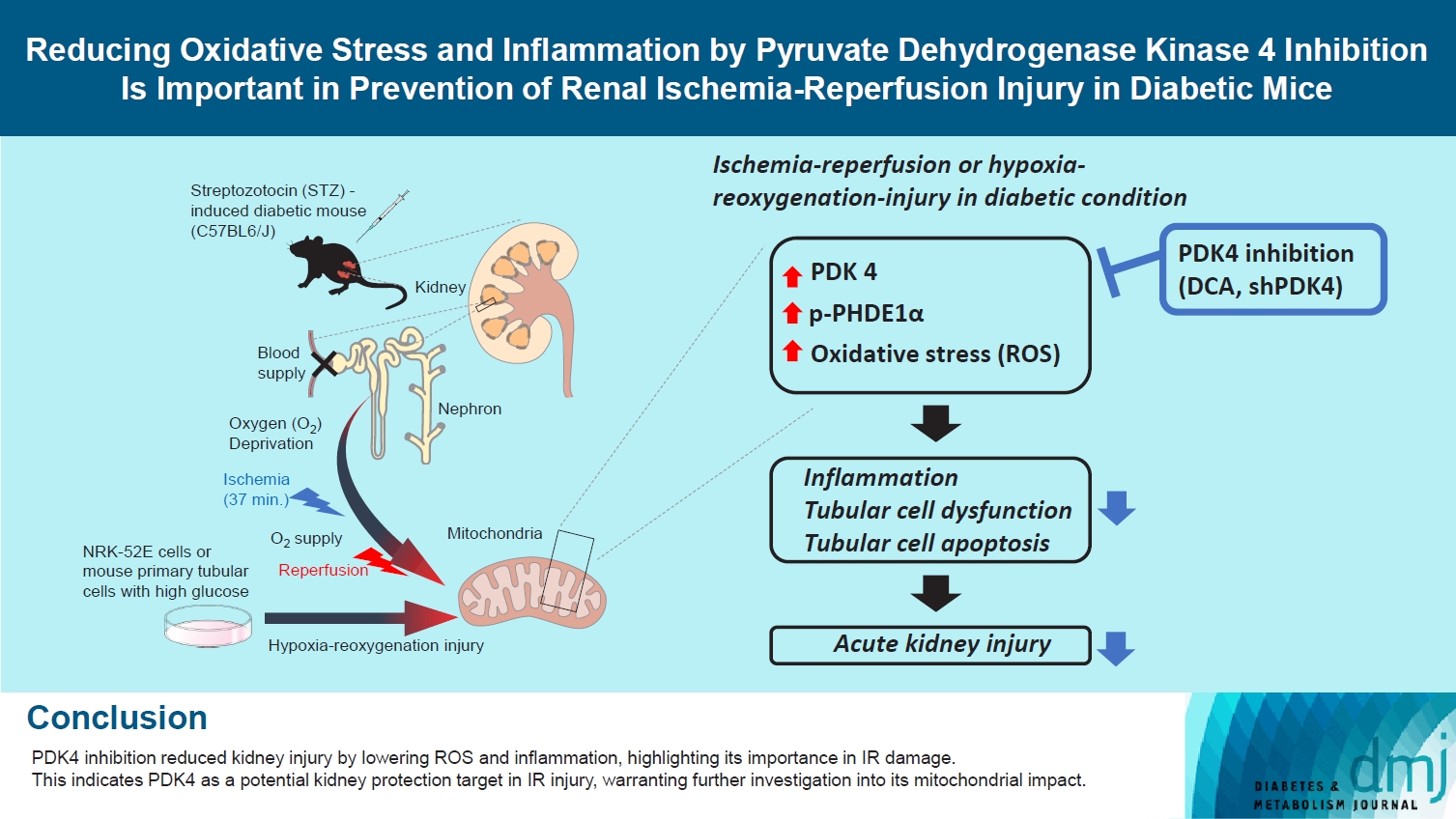
Reactive oxygen species (ROS) and inflammation are reported to have a fundamental role in the pathogenesis of ischemia-reperfusion (IR) injury, a leading cause of acute kidney injury. The present study investigated the role of pyruvate dehydrogenase kinase 4 (PDK4) in ROS production and inflammation following IR injury.
Methods
We used a streptozotocin-induced diabetic C57BL6/J mouse model, which was subjected to IR by clamping both renal pedicles. Cellular apoptosis and inflammatory markers were evaluated in NRK-52E cells and mouse primary tubular cells after hypoxia and reoxygenation using a hypoxia work station.
Results
Following IR injury in diabetic mice, the expression of PDK4, rather than the other PDK isoforms, was induced with a marked increase in pyruvate dehydrogenase E1α (PDHE1α) phosphorylation. This was accompanied by a pronounced ROS activation, as well as tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), interleukin-1β (IL-1β), and monocyte chemoattractant protein-1 (MCP-1) production. Notably, sodium dichloroacetate (DCA) attenuated renal IR injury-induced apoptosis which can be attributed to reducing PDK4 expression and PDHE1α phosphorylation levels. DCA or shPdk4 treatment reduced oxidative stress and decreased TNF-α, IL-6, IL-1β, and MCP-1 production after IR or hypoxia-reoxygenation injury.
Conclusion
PDK4 inhibition alleviated renal injury with decreased ROS production and inflammation, supporting a critical role for PDK4 in IR mediated damage. This result indicates another potential target for reno-protection during IR injury; accordingly, the role of PDK4 inhibition needs to be comprehensively elucidated in terms of mitochondrial function during renal IR injury.
- Basic Research
- CycloZ Improves Hyperglycemia and Lipid Metabolism by Modulating Lysine Acetylation in KK-Ay Mice
- Jongsu Jeon, Dohyun Lee, Bobae Kim, Bo-Yoon Park, Chang Joo Oh, Min-Ji Kim, Jae-Han Jeon, In-Kyu Lee, Onyu Park, Seoyeong Baek, Chae Won Lim, Dongryeol Ryu, Sungsoon Fang, Johan Auwerx, Kyong-Tai Kim, Hoe-Yune Jung
- Diabetes Metab J. 2023;47(5):653-667. Published online April 26, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0244

- 2,731 View
- 193 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
CycloZ, a combination of cyclo-His-Pro and zinc, has anti-diabetic activity. However, its exact mode of action remains to be elucidated.
Methods
KK-Ay mice, a type 2 diabetes mellitus (T2DM) model, were administered CycloZ either as a preventive intervention, or as a therapy. Glycemic control was evaluated using the oral glucose tolerance test (OGTT), and glycosylated hemoglobin (HbA1c) levels. Liver and visceral adipose tissues (VATs) were used for histological evaluation, gene expression analysis, and protein expression analysis.
Results
CycloZ administration improved glycemic control in KK-Ay mice in both prophylactic and therapeutic studies. Lysine acetylation of peroxisome proliferator-activated receptor gamma coactivator 1-alpha, liver kinase B1, and nuclear factor-κB p65 was decreased in the liver and VATs in CycloZ-treated mice. In addition, CycloZ treatment improved mitochondrial function, lipid oxidation, and inflammation in the liver and VATs of mice. CycloZ treatment also increased the level of β-nicotinamide adenine dinucleotide (NAD+), which affected the activity of deacetylases, such as sirtuin 1 (Sirt1).
Conclusion
Our findings suggest that the beneficial effects of CycloZ on diabetes and obesity occur through increased NAD+ synthesis, which modulates Sirt1 deacetylase activity in the liver and VATs. Given that the mode of action of an NAD+ booster or Sirt1 deacetylase activator is different from that of traditional T2DM drugs, CycloZ would be considered a novel therapeutic option for the treatment of T2DM.
- Basic Research
- The Link between Mitochondrial Dysfunction and Sarcopenia: An Update Focusing on the Role of Pyruvate Dehydrogenase Kinase 4
- Min-Ji Kim, Ibotombi Singh Sinam, Zerwa Siddique, Jae-Han Jeon, In-Kyu Lee
- Diabetes Metab J. 2023;47(2):153-163. Published online January 12, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0305
- 4,858 View
- 367 Download
- 5 Web of Science
- 6 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
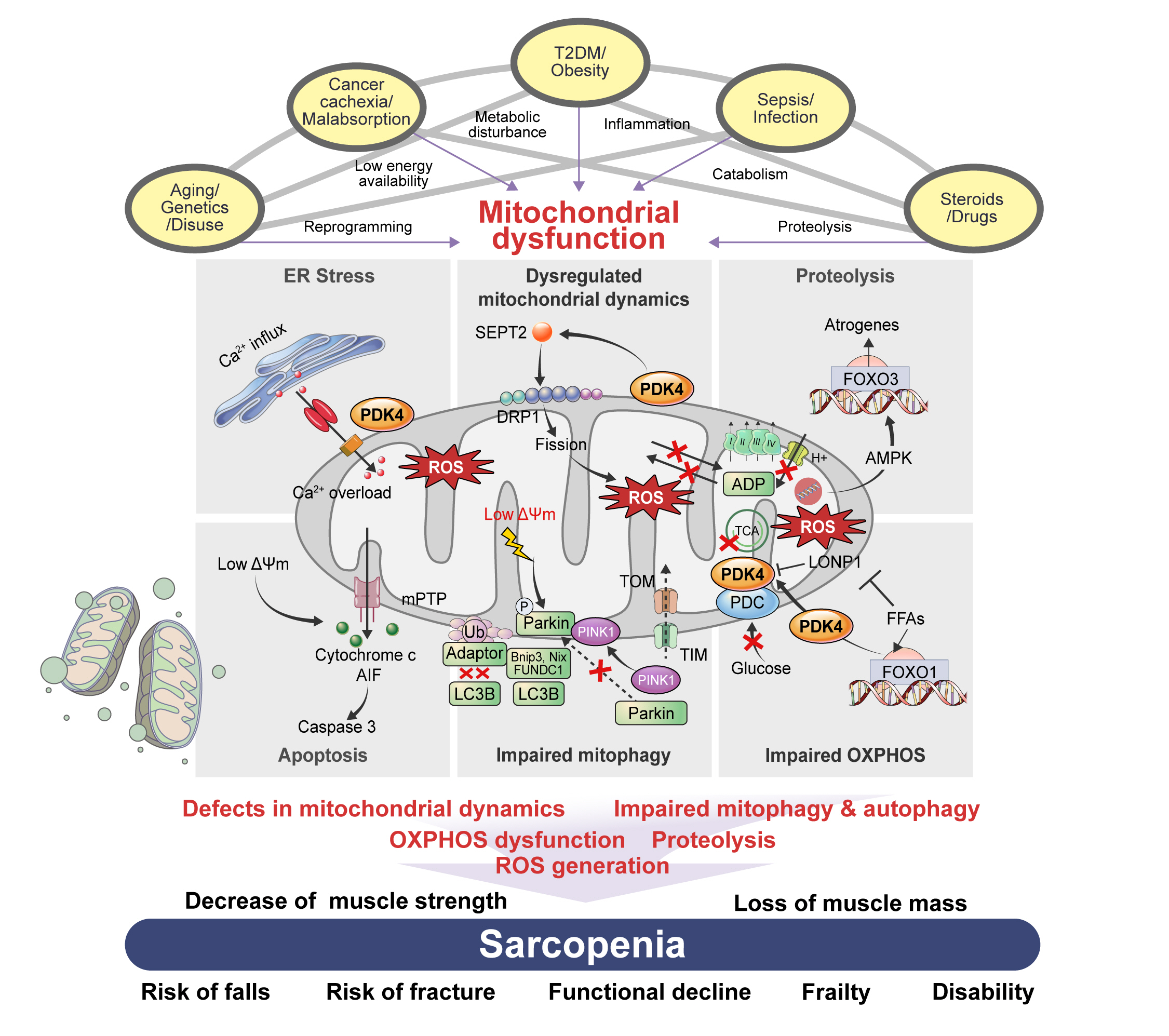
ePub - Sarcopenia, defined as a progressive loss of muscle mass and function, is typified by mitochondrial dysfunction and loss of mitochondrial resilience. Sarcopenia is associated not only with aging, but also with various metabolic diseases characterized by mitochondrial dyshomeostasis. Pyruvate dehydrogenase kinases (PDKs) are mitochondrial enzymes that inhibit the pyruvate dehydrogenase complex, which controls pyruvate entry into the tricarboxylic acid cycle and the subsequent adenosine triphosphate production required for normal cellular activities. PDK4 is upregulated in mitochondrial dysfunction-related metabolic diseases, especially pathologic muscle conditions associated with enhanced muscle proteolysis and aberrant myogenesis. Increases in PDK4 are associated with perturbation of mitochondria-associated membranes and mitochondrial quality control, which are emerging as a central mechanism in the pathogenesis of metabolic disease-associated muscle atrophy. Here, we review how mitochondrial dysfunction affects sarcopenia, focusing on the role of PDK4 in mitochondrial homeostasis. We discuss the molecular mechanisms underlying the effects of PDK4 on mitochondrial dysfunction in sarcopenia and show that targeting mitochondria could be a therapeutic target for treating sarcopenia.
-
Citations
Citations to this article as recorded by- Synthesis, activatory effects, molecular docking and ADME studies as rabbit muscle pyruvate kinase activators of ureido phenyl substituted 1,4-dihydropyridine derivatives
Mustafa Oğuzhan Kaya, Tuna Demirci, Ümit Çalışır, Oğuzhan Özdemir, Yeşim Kaya, Mustafa Arslan
Research on Chemical Intermediates.2024; 50(1): 437. CrossRef - Unraveling the causes of sarcopenia: Roles of neuromuscular junction impairment and mitochondrial dysfunction
Yanmei Miao, Leiyu Xie, Jiamei Song, Xing Cai, Jinghe Yang, Xinglong Ma, Shaolin Chen, Peng Xie
Physiological Reports.2024;[Epub] CrossRef - Metabolic clues to aging: exploring the role of circulating metabolites in frailty, sarcopenia and vascular aging related traits and diseases
Zonghao Qian, Yuzhen Huang, Yucong Zhang, Ni Yang, Ziwei Fang, Cuntai Zhang, Le Zhang
Frontiers in Genetics.2024;[Epub] CrossRef - Inhibition of Pyruvate Dehydrogenase Kinase 4 Protects Cardiomyocytes from lipopolysaccharide-Induced Mitochondrial Damage by Reducing Lactate Accumulation
Tangtian Chen, Qiumin Xie, Bin Tan, Qin Yi, Han Xiang, Rui Wang, Qin Zhou, Bolin He, Jie Tian, Jing Zhu, Hao Xu
Inflammation.2024;[Epub] CrossRef - Effect of resistance training plus enriched probiotic supplement on sestrin2, oxidative stress, and mitophagy markers in elderly male Wistar rats
Majid Mohabbat, Hamid Arazi
Scientific Reports.2024;[Epub] CrossRef - Neuroprotective Effects and Therapeutic Potential of Dichloroacetate: Targeting Metabolic Disorders in Nervous System Diseases
Yue Zhang, Meiyan Sun, Hongxiang Zhao, Zhengyan Wang, Yanan Shi, Jianxin Dong, Kaifang Wang, Xi Wang, Xingyue Li, Haiyan Qi, Xiaoyong Zhao
International Journal of Nanomedicine.2023; Volume 18: 7559. CrossRef
- Synthesis, activatory effects, molecular docking and ADME studies as rabbit muscle pyruvate kinase activators of ureido phenyl substituted 1,4-dihydropyridine derivatives
- Impact of Social Distancing Due to Coronavirus Disease 2019 on the Changes in Glycosylated Hemoglobin Level in People with Type 2 Diabetes Mellitus (Diabetes Metab J 2021;45:109-14)
- Sung-Don Park, Sung-Woo Kim, Jun Sung Moon, Jae-Han Jeon, Mi Kyung Kim, Keun-Gyu Park
- Diabetes Metab J. 2021;45(2):279-280. Published online March 25, 2021
- DOI: https://doi.org/10.4093/dmj.2020.0300
- 4,212 View
- 87 Download
- 1 Crossref
-
 PDF
PDF PubReader
PubReader  ePub
ePub -
Citations
Citations to this article as recorded by- A cross-sectional study on the telemedicine usage and glycemic status of diabetic patients during the COVID-19 pandemic
Novi Sulistia Wati, Pokkate Wongsasuluk, Pradana Soewondo
Medical Journal of Indonesia.2021; 30(3): 215. CrossRef
- A cross-sectional study on the telemedicine usage and glycemic status of diabetic patients during the COVID-19 pandemic
- COVID-19
- Impact of Social Distancing Due to Coronavirus Disease 2019 on the Changes in Glycosylated Hemoglobin Level in People with Type 2 Diabetes Mellitus
- Sung-Don Park, Sung-Woo Kim, Jun Sung Moon, Yin Young Lee, Nan Hee Cho, Ji-Hyun Lee, Jae-Han Jeon, Yeon-Kyung Choi, Mi Kyung Kim, Keun-Gyu Park
- Diabetes Metab J. 2021;45(1):109-114. Published online December 4, 2020
- DOI: https://doi.org/10.4093/dmj.2020.0226
- 9,421 View
- 307 Download
- 23 Web of Science
- 24 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub 
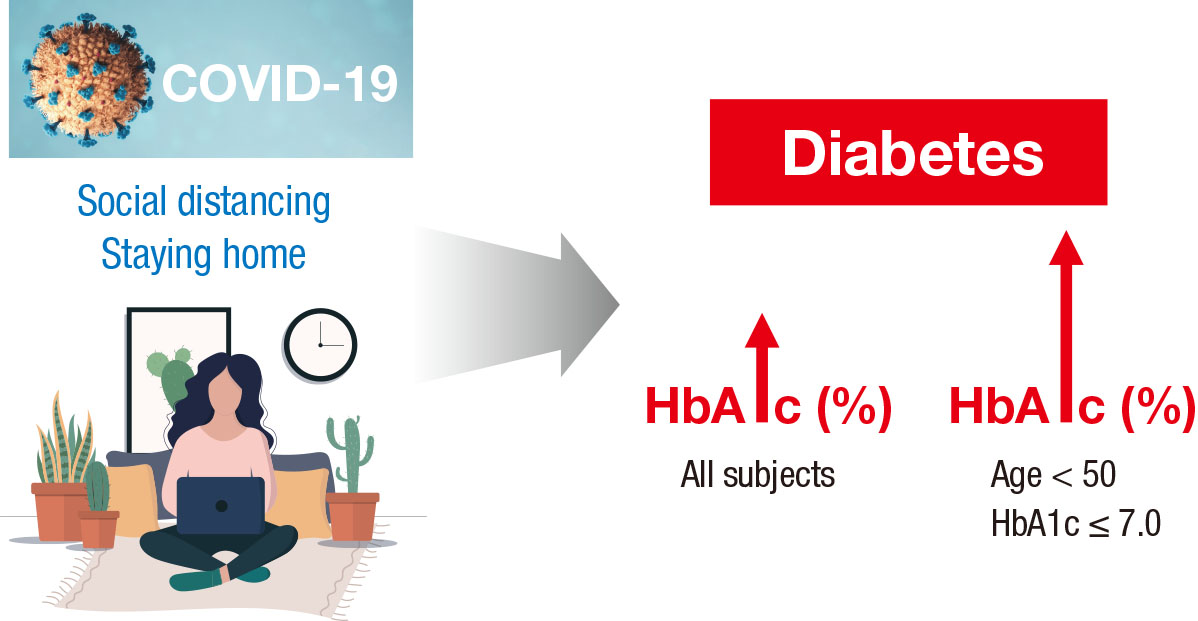
- This study investigated the impact of social distancing due to coronavirus disease 2019 (COVID-19) on glycemic control in people with type 2 diabetes mellitus (T2DM). We retrospectively analyzed the change in glycosylated hemoglobin level (ΔHbA1c) in people with T2DM who undertook social distancing because of COVID-19. We compared the ΔHbA1c between COVID-19 and non-COVID-19 cohorts that were enrolled at the same time of year. The ΔHbA1c of the COVID-19 cohort was significantly higher than that of two non-COVID-19 cohorts. Subgroup analysis according to age and baseline HbA1c level showed that social distancing significantly increased the mean HbA1c level of participants of <50 years. The ΔHbA1c of participants of <50 years and with HbA1c <7.0% in the COVID-19 cohort showed larger changes than other subgroups. In adjusted model, adjusted ΔHbA1c levels in the COVID-19 cohort remained significantly higher than those in the two other cohorts. Social distancing negatively impacts blood glucose control in people with T2DM, especially those who are younger and have good blood glucose control.
-
Citations
Citations to this article as recorded by- Impact of two COVID-19 lockdowns on HbA1c levels in patients with type 2 diabetes and associations with patient characteristics: a multicentre, observational cohort study over three years
Ingmar Schäfer, Daniel Tajdar, Laura Walther, Lasse Bittner, Dagmar Lühmann, Martin Scherer
Frontiers in Public Health.2024;[Epub] CrossRef - Influence of the COVID-19 pandemic on the achievement of guideline targets for HbA1c, blood pressure, and LDL cholesterol in people with diabetes in Japan
Shingo Kuwajima, Takahito Itoh, Tatsuya Sato, Shoya Ino, Satoru Shibata, Kouhei Ohno, Hiroyuki Hotta, Tomoaki Matsumoto, Hitoshi Ooiwa, Hirofumi Kubo, Takayuki Miki
Diabetology International.2024;[Epub] CrossRef - Socioeconomic status and the effect of prolonged pandemic confinement on anthropometric and glycaemic outcomes in adults with type 2 diabetes mellitus
Chandana Wijeweera, Ummul Muhfaza, Reginald V. Lord, Peter Petocz, Juliana Chen, Veronica Preda
Primary Care Diabetes.2024;[Epub] CrossRef - Physical and Mental Health Characteristics of Hospitalized COVID-19 Patients with and without Type 2 Diabetes Mellitus in Turkey
Abdulbari Bener, Murat Atmaca, Abdulla O. A. A. Al-Hamaq, Antonio Ventriglio
Brain Sciences.2024; 14(4): 377. CrossRef - Self-Care of Adults with Type 2 Diabetes During the COVID-19 Pandemic: A Qualitative Interpretive Description Study
Michela Luciani, Camilla Bigoni, Marta Canesi, Matteo Masotto, Diletta Fabrizi, Stefania Di Mauro, Davide Ausili
Clinical Nursing Research.2023; 32(1): 73. CrossRef - Changes in body weight and glycemic control in association with COVID-19 Shutdown among 23,000 adults with type 2 diabetes
Emily Panza, Kevin E. Kip, Kripa Venkatakrishnan, Oscar C. Marroquin, Rena R. Wing
Acta Diabetologica.2023; 60(6): 787. CrossRef - The Impact of a Lockdown for the COVID-19 Pandemic on Seasonal HbA1c Variation in Patients with Type 2 Diabetes
Yu-Cheng Cheng, Yu-Hsuan Li, Hsiu-Chen Liu, Chiann-Yi Hsu, Wan-Jen Chang, I-Te Lee, Chin-Li Lu
Life.2023; 13(3): 763. CrossRef - Changes in the mean incidence and variance of orthopedic diseases before and during the COVID-19 pandemic in Korea: a retrospective study
Joo-Hee Kim, Mi Jung Kwon, Hyo Geun Choi, Sang Jun Lee, Sangwon Hwang, Jaemin Lee, San-Hui Lee, Jung Woo Lee
BMC Musculoskeletal Disorders.2023;[Epub] CrossRef - Gender differences-based bioinformatics analysis to identify hub genes and key pathways in type 2 diabetes
Md Sojib Hossain, Subrina Islam Rupa, Md Sumon Sarkar, Md Al Amin, Mst Tania Khatun, Md Shamim, Md Zahidul Islam
Informatics in Medicine Unlocked.2023; 40: 101302. CrossRef - Retrospective Study on the Impact of COVID-19 Lockdown on Patients with Type 2 Diabetes in Northern Taiwan
Hsuan Huang, Hsiao-Ling Su, Chih-Hsung Huang, Yi-Hsin Lin
Diabetes, Metabolic Syndrome and Obesity.2023; Volume 16: 2539. CrossRef - Understanding impacts of COVID-19 restrictions on glycemic control for patients with diabetes in Japan
Kiyoko Uno-Eder, Noriko Satoh-Asahara, Manabu Hibiya, Kenji Uno, Takuya Uchino, Koji Morita, Toshio Ishikawa, Tetsuji Kaneko, Hajime Yamakage, Yuki Kitaoka, Tomohiro Sawa, Kazuhisa Tsukamoto, Tamio Teramoto
Journal of Diabetes & Metabolic Disorders.2023; 22(2): 1695. CrossRef - Impacts of the COVID-19 pandemic on unmet social needs, self-care, and outcomes among people with diabetes and poor glycemic control
Minal R. Patel, Guanghao Zhang, Cindy Leung, Peter X.K. Song, Michele Heisler, Hae Mi Choe, Roshanak Mehdipanah, Xu Shi, Kenneth Resnicow, Geila Rajaee, John D. Piette
Primary Care Diabetes.2022; 16(1): 57. CrossRef - Impact of the COVID-19 Pandemic on Glycemic Control and Blood Pressure Control in Patients with Diabetes in Japan
Keisuke Endo, Takayuki Miki, Takahito Itoh, Hirofumi Kubo, Ryosuke Ito, Kouhei Ohno, Hiroyuki Hotta, Nobuo Kato, Tomoaki Matsumoto, Aya Kitamura, Mai Tamayama, Takako Wataya, Ayaka Yamaya, Rei Ishikawa, Hitoshi Ooiwa
Internal Medicine.2022; 61(1): 37. CrossRef - The Effects of COVID-19 Lockdown on Glycaemic Control and Lipid Profile in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis
Omorogieva Ojo, Xiao-Hua Wang, Osarhumwese Osaretin Ojo, Edith Orjih, Nivedita Pavithran, Amanda Rodrigues Amorim Adegboye, Qian-Qian Feng, Paul McCrone
International Journal of Environmental Research and Public Health.2022; 19(3): 1095. CrossRef - Lifestyles Under Lockdown: A Scoping Review of International Studies on Type 2 Diabetes Self-Management Behaviors During COVID-19
Caroline Cummings, Kagnica Seng, Ryan Tweet, Julie Wagner
Frontiers in Clinical Diabetes and Healthcare.2022;[Epub] CrossRef - Substitution of telemedicine for clinic visit during the COVID‐19 pandemic of 2020: Comparison of telemedicine and clinic visit
Yukiko Onishi, Rieko Ichihashi, Yoko Yoshida, Tazu Tahara, Takako Kikuchi, Toshiko Kobori, Tetsuya Kubota, Masahiko Iwamoto, Shoko Hamano, Masato Kasuga
Journal of Diabetes Investigation.2022; 13(9): 1617. CrossRef - The impact of the COVID-19 pandemic on the management of patients with chronic diseases in Primary Health Care
Panagiotis Stachteas, Manolis Symvoulakis, Apostolos Tsapas, Emmanouil Smyrnakis
Population Medicine.2022; 4(August): 1. CrossRef - Effects of COVID-19 Pandemic and Lockdown on Monitoring and Treatment Balance of Finnish Coronary Heart Disease and Type 2 Diabetes Patients
Piia Lavikainen, Marja-Leena Lamidi, Teppo Repo, Laura Inglin, Janne Martikainen, Tiina Laatikainen
Clinical Epidemiology.2022; Volume 14: 1363. CrossRef - Impact of Social Distancing Due to Coronavirus Disease 2019 on the Changes in Glycosylated Hemoglobin Level in People with Type 2 Diabetes Mellitus (Diabetes Metab J 2021;45:109-14)
Junghyun Noh
Diabetes & Metabolism Journal.2021; 45(2): 275. CrossRef - Impact of Social Distancing Due to Coronavirus Disease 2019 on the Changes in Glycosylated Hemoglobin Level in People with Type 2 Diabetes Mellitus (Diabetes Metab J 2021;45:109-14)
Sung-Don Park, Sung-Woo Kim, Jun Sung Moon, Jae-Han Jeon, Mi Kyung Kim, Keun-Gyu Park
Diabetes & Metabolism Journal.2021; 45(2): 279. CrossRef - Glucose control in diabetes during home confinement for the first pandemic wave of COVID-19: a meta-analysis of observational studies
Giovanni Antonio Silverii, Chiara Delli Poggi, Ilaria Dicembrini, Matteo Monami, Edoardo Mannucci
Acta Diabetologica.2021; 58(12): 1603. CrossRef - The impact of COVID-19 pandemic on glycemic control in patients with diabetes mellitus in Turkey: a multi-center study from Kocaeli
Alev Selek, Emre Gezer, Eda Altun, Mehmet Sözen, Ömercan Topaloğlu, Damla Köksalan, Halil Demirkan, Dilek Karakaya, Berrin Cetinarslan, Zeynep Cantürk, Dilek Taymez
Journal of Diabetes & Metabolic Disorders.2021; 20(2): 1461. CrossRef - Effects of Social Distancing on Diabetes Management in Older Adults during COVID-19 Pandemic
Soo Myoung Shin, Tae Jung Oh, Sung Hee Choi, Hak Chul Jang
Diabetes & Metabolism Journal.2021; 45(5): 765. CrossRef - Year-Long Trend in Glycated Hemoglobin Levels in Patients with Type 2 Diabetes during the COVID-19 Pandemic
Jonghwa Jin, Seong Wook Lee, Won-Ki Lee, Jae-Han Jeon, Jung-Guk Kim, In-Kyu Lee, Yeon-Kyung Choi, Keun-Gyu Park
Endocrinology and Metabolism.2021; 36(5): 1142. CrossRef
- Impact of two COVID-19 lockdowns on HbA1c levels in patients with type 2 diabetes and associations with patient characteristics: a multicentre, observational cohort study over three years
- Covid-19
-
- The Clinical Characteristics and Outcomes of Patients with Moderate-to-Severe Coronavirus Disease 2019 Infection and Diabetes in Daegu, South Korea
- Mi Kyung Kim, Jae-Han Jeon, Sung-Woo Kim, Jun Sung Moon, Nan Hee Cho, Eugene Han, Ji Hong You, Ji Yeon Lee, Miri Hyun, Jae Seok Park, Yong Shik Kwon, Yeon-Kyung Choi, Ki Tae Kwon, Shin Yup Lee, Eon Ju Jeon, Jin-Woo Kim, Hyo-Lim Hong, Hyun Hee Kwon, Chi Young Jung, Yin Young Lee, Eunyeoung Ha, Seung Min Chung, Jian Hur, June Hong Ahn, Na-young Kim, Shin-Woo Kim, Hyun Ha Chang, Yong Hoon Lee, Jaehee Lee, Keun-Gyu Park, Hyun Ah Kim, Ji-Hyun Lee
- Diabetes Metab J. 2020;44(4):602-613. Published online August 12, 2020
- DOI: https://doi.org/10.4093/dmj.2020.0146

- 13,305 View
- 206 Download
- 67 Web of Science
- 74 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub Background Coronavirus disease 2019 (COVID-19) is a global pandemic that had affected more than eight million people worldwide by June 2020. Given the importance of the presence of diabetes mellitus (DM) for host immunity, we retrospectively evaluated the clinical characteristics and outcomes of moderate-to-severe COVID-19 in patients with diabetes.
Methods We conducted a multi-center observational study of 1,082 adult inpatients (aged ≥18 years) who were admitted to one of five university hospitals in Daegu because of the severity of their COVID-19-related disease. The demographic, laboratory, and radiologic findings, and the mortality, prevalence of severe disease, and duration of quarantine were compared between patients with and without DM. In addition, 1:1 propensity score (PS)-matching was conducted with the DM group.
Results Compared with the non-DM group (
n =847), patients with DM (n =235) were older, exhibited higher mortality, and required more intensive care. Even after PS-matching, patients with DM exhibited more severe disease, and DM remained a prognostic factor for higher mortality (hazard ratio, 2.40; 95% confidence interval, 1.38 to 4.15). Subgroup analysis revealed that the presence of DM was associated with higher mortality, especially in older people (≥70 years old). Prior use of a dipeptidyl peptidase-4 inhibitor or a renin-angiotensin system inhibitor did not affect mortality or the clinical severity of the disease.Conclusion DM is a significant risk factor for COVID-19 severity and mortality. Our findings imply that COVID-19 patients with DM, especially if elderly, require special attention and prompt intensive care.
-
Citations
Citations to this article as recorded by- Potential use of sodium glucose co-transporter 2 inhibitors during acute illness: a systematic review based on COVID-19
Carmen Tisch, Eleni Xourgia, Aristomenis Exadaktylos, Mairi Ziaka
Endocrine.2024;[Epub] CrossRef - Insulin and Metformin Administration: Unravelling the Multifaceted Association with Mortality across Various Clinical Settings Considering Type 2 Diabetes Mellitus and COVID-19
Łukasz Lewandowski, Agnieszka Bronowicka-Szydełko, Maciej Rabczyński, Dorota Bednarska-Chabowska, Joanna Adamiec-Mroczek, Adrian Doroszko, Małgorzata Trocha, Krzysztof Kujawa, Agnieszka Matera-Witkiewicz, Edwin Kuźnik, Paweł Lubieniecki, Marcin Madziarski
Biomedicines.2024; 12(3): 605. CrossRef - Pre-admission use of sodium glucose transporter-2 inhibitor (SGLT-2i) may significantly improves Covid-19 outcomes in patients with diabetes: A systematic review, meta-analysis, and meta-regression
Hikmat Permana, Theo Audi Yanto, Timotius Ivan Hariyanto
Diabetes Research and Clinical Practice.2023; 195: 110205. CrossRef - Risk phenotypes of diabetes and association with COVID-19 severity and death: an update of a living systematic review and meta-analysis
Sabrina Schlesinger, Alexander Lang, Nikoletta Christodoulou, Philipp Linnerz, Kalliopi Pafili, Oliver Kuss, Christian Herder, Manuela Neuenschwander, Janett Barbaresko, Michael Roden
Diabetologia.2023; 66(8): 1395. CrossRef - Factors influencing the severity of COVID-19 course for patients with diabetes mellitus in tashkent: a retrospective cohort study
A. V. Alieva, A. A. Djalilov, F. A. Khaydarova, A. V. Alimov, D. Z. Khalilova, V. A. Talenova, N. U. Alimova, M. D. Aripova, A. S. Sadikova
Obesity and metabolism.2023; 20(2): 92. CrossRef - Pituitary Diseases and COVID-19 Outcomes in South Korea: A Nationwide Cohort Study
Jeonghoon Ha, Kyoung Min Kim, Dong-Jun Lim, Keeho Song, Gi Hyeon Seo
Journal of Clinical Medicine.2023; 12(14): 4799. CrossRef - Epidemiological features and consequences of COVID‐19 in patients with and without gastrointestinal symptoms in southwestern Iran. A retrospective observational study
Habibollah Azarbakhsh, Leila Moftakhar, Aliasghar Valipour, Alireza Mirahmadizadeh, Hekmat Allah Moradi, Elahe Piraee
Health Science Reports.2023;[Epub] CrossRef - The Impact of Long-Term Conditions and Comorbidity Patterns on COVID-19 Infection and Hospitalisation: A Cohort Study
Yun-Ting Huang, Andrew Steptoe, Riyaz S. Patel, Esme Fuller Thomson, Dorina Cadar
Gerontology.2023; 69(10): 1200. CrossRef - Association Between Anti-diabetic Agents and Clinical Outcomes of COVID-19 in Patients with Diabetes: A Systematic Review and Meta-Analysis
Tiantian Han, Shaodi Ma, Chenyu Sun, Huimei Zhang, Guangbo Qu, Yue Chen, Ce Cheng, Eric L. Chen, Mubashir Ayaz Ahmed, Keun Young Kim, Raveena Manem, Mengshi Chen, Zhichun Guo, Hongru Yang, Yue Yan, Qin Zhou
Archives of Medical Research.2022; 53(2): 186. CrossRef - Use of DPP4i reduced odds of clinical deterioration and hyperinflammatory syndrome in COVID-19 patients with type 2 diabetes: Propensity score analysis of a territory-wide cohort in Hong Kong
Carlos K.H. Wong, David T.W. Lui, Angel Y.C. Lui, Ashley C.Y. Kwok, Marshall C.H. Low, Kristy T.K. Lau, Ivan C.H. Au, Xi Xiong, Matthew S.H. Chung, Eric H.Y. Lau, Benjamin J. Cowling
Diabetes & Metabolism.2022; 48(1): 101307. CrossRef - Dipeptidyl peptidase-4 (DPP-IV) inhibitor was associated with mortality reduction in COVID-19 — A systematic review and meta-analysis
Ahmad Fariz Malvi Zamzam Zein, Wilson Matthew Raffaello
Primary Care Diabetes.2022; 16(1): 162. CrossRef - Prevalence and impact of diabetes in hospitalized COVID‐19 patients: A systematic review and meta‐analysis
Sian A. Bradley, Maciej Banach, Negman Alvarado, Ivica Smokovski, Sonu M. M. Bhaskar
Journal of Diabetes.2022; 14(2): 144. CrossRef - Interplay between Inflammaging, Frailty and Nutrition in Covid-19: Preventive and Adjuvant Treatment Perspectives
A. Padilha de Lima, M. Macedo Rogero, T. Araujo Viel, H.M. Garay-Malpartida, I. Aprahamian, Sandra Maria Lima Ribeiro
The Journal of nutrition, health and aging.2022; 26(1): 67. CrossRef - Increase in blood glucose level and incidence of diabetic ketoacidosis in children with type 1 diabetes mellitus in the Daegu-Gyeongbuk area during the coronavirus disease 2019 (COVID-19) pandemic: a retrospective cross-sectional study
Mi Seon Lee, Rosie Lee, Cheol Woo Ko, Jung Eun Moon
Journal of Yeungnam Medical Science.2022; 39(1): 46. CrossRef - Interrelationship between 2019-nCov receptor DPP4 and diabetes mellitus targets based on protein interaction network
Qian Gao, Wenjun Zhang, Tingting Li, Guojun Yang, Wei Zhu, Naijun Chen, Huawei Jin
Scientific Reports.2022;[Epub] CrossRef - Can sodium-glucose co-transporter-2 (SGLT-2) inhibitor reduce the risk of adverse complications due to COVID-19? – Targeting hyperinflammation
Afnan Alshnbari, Iskandar Idris
Current Medical Research and Opinion.2022; 38(3): 357. CrossRef - Commentary: Mortality Risk of Antidiabetic Agents for Type 2 Diabetes With COVID-19: A Systematic Review and Meta-Analysis
Li-Min Zhao, Xie-Hui Chen, Mei Qiu
Frontiers in Endocrinology.2022;[Epub] CrossRef - COVID-19 and Diabetes
Awadhesh Kumar Singh, Kamlesh Khunti
Annual Review of Medicine.2022; 73(1): 129. CrossRef - The enzymes in COVID-19: A review
Maria Helena Menezes Estevam Alves, Layla Carvalho Mahnke, Tifany Cerqueira Macedo, Thais Ketinly dos Santos Silva, Luiz Bezerra Carvalho Junior
Biochimie.2022; 197: 38. CrossRef - IMPACT OF ANTIDIABETIC DRUGS ON RISK AND OUTCOME OF COVID-19 INFECTION: A REVIEW
Adnan A. Zainal, Marwan M. Merkhan
Military Medical Science Letters.2022; 91(2): 140. CrossRef - Does metformin affect outcomes in COVID‐19 patients with new or pre‐existing diabetes mellitus? A systematic review and meta‐analysis
Adithan Ganesh, Michael D. Randall
British Journal of Clinical Pharmacology.2022; 88(6): 2642. CrossRef - Diabetes, Metformin and the Clinical Course of Covid-19: Outcomes, Mechanisms and Suggestions on the Therapeutic Use of Metformin
Clifford J. Bailey, Mike Gwilt
Frontiers in Pharmacology.2022;[Epub] CrossRef - The Role of Diabetes and Hyperglycemia on COVID-19 Infection Course—A Narrative Review
Evangelia Tzeravini, Eleftherios Stratigakos, Chris Siafarikas, Anastasios Tentolouris, Nikolaos Tentolouris
Frontiers in Clinical Diabetes and Healthcare.2022;[Epub] CrossRef - Preadmission use of antidiabetic medications and mortality among patients with COVID-19 having type 2 diabetes: A meta-analysis
Nam Nhat Nguyen, Dung Si Ho, Hung Song Nguyen, Dang Khanh Ngan Ho, Hung-Yuan Li, Chia-Yuan Lin, Hsiao-Yean Chiu, Yang-Ching Chen
Metabolism.2022; 131: 155196. CrossRef - Glucose-Lowering Agents and COVID-19
Ah Reum Khang
The Journal of Korean Diabetes.2022; 23(1): 1. CrossRef - Impact of diabetes on COVID‐19 mortality and hospital outcomes from a global perspective: An umbrella systematic review and meta‐analysis
Stavroula Kastora, Manisha Patel, Ben Carter, Mirela Delibegovic, Phyo Kyaw Myint
Endocrinology, Diabetes & Metabolism.2022;[Epub] CrossRef - The Association Between Antidiabetic Agents and Clinical Outcomes of COVID-19 Patients With Diabetes: A Bayesian Network Meta-Analysis
Yidan Chen, Xingfei Lv, Sang Lin, Mohammad Arshad, Mengjun Dai
Frontiers in Endocrinology.2022;[Epub] CrossRef - Renin‐Angiotensin Aldosterone System Inhibitors and COVID‐19: A Systematic Review and Meta‐Analysis Revealing Critical Bias Across a Body of Observational Research
Jordan Loader, Frances C. Taylor, Erik Lampa, Johan Sundström
Journal of the American Heart Association.2022;[Epub] CrossRef - Diabetes and SARS-CoV-2–Is There a Mutual Connection?
Anna P. Jedrzejak, Edyta K. Urbaniak, Jadwiga A. Wasko, Natalia Ziojla, Malgorzata Borowiak
Frontiers in Cell and Developmental Biology.2022;[Epub] CrossRef - The relationship of age, sex and prothrombin time related to the severity and mortality of COVID-19 patients with diabetes mellitus: a systematic review and meta analysis
Audrey Fabianisa Mirza, Ceria Halim, Mutiara Indah Sari
F1000Research.2022; 11: 729. CrossRef - Are lipid ratios and triglyceride-glucose index associated with critical care outcomes in COVID-19 patients?
Marzieh Rohani-Rasaf, Kosar Mirjalili, Akram Vatannejad, Maryam Teimouri, Xiao-Feng Yang
PLOS ONE.2022; 17(8): e0272000. CrossRef - Early glycaemic variability increases 28-day mortality and prolongs intensive care unit stay in critically ill patients with pneumonia
Seong Ho Kim, Ji Young Kim, Eun Song Kim, Il Rae Park, Eun Yeong Ha, Seung Min Chung, Jun Sung Moon, Ji Sung Yoon, Kyu Chang Won, Hyoung Woo Lee
Annals of Medicine.2022; 54(1): 2724. CrossRef - Dipeptidyl peptidase 4 inhibitors in COVID-19: Beyond glycemic control
Niya Narayanan, Dukhabandhu Naik, Jayaprakash Sahoo, Sadishkumar Kamalanathan
World Journal of Virology.2022; 11(6): 399. CrossRef - Prevalencia de secuelas en pacientes con diabetes mellitus tipo 2 sobrevivientes al COVID-19
Gianela M. Cancino-Castillo, Miguel A. Tresierra-Ayala, Jorge L. Campos-Reyna, Jaime Rosales-Rimache
REVISTA MÉDICA VALLEJIANA/ Vallejian Medical Journal.2022; 11(2): 48. CrossRef - Predictors of adverse in-hospital outcome and recovery in patients with diabetes mellitus and COVID-19 pneumonia in Iraq
Hussein Nafakhi, Mohammed Alareedh, Karrar Al-Buthabhak, Foaad Shaghee, Ahmed Nafakhi, Samet Kasim
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2021; 15(1): 33. CrossRef - Non-insulin anti-diabetic agents in patients with type 2 diabetes and COVID-19: A Critical Appraisal of Literature
Awadhesh Kumar Singh, Ritu Singh, Banshi Saboo, Anoop Misra
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2021; 15(1): 159. CrossRef - COVID-19 associated with diabetes and other noncommunicable diseases led to a global health crisis
Mark Thomaz Ugliara Barone, Belinda Ngongo, Simone Bega Harnik, Lucas Xavier de Oliveira, Dániel Végh, Patrícia Vieira de Luca, Hermelinda Cordeiro Pedrosa, Franco Giraudo, Roque Cardona-Hernandez, Nayanjeet Chaudhury, Luiz Menna-Barreto
Diabetes Research and Clinical Practice.2021; 171: 108587. CrossRef - A meta-analysis on the preadmission use of DPP-4 inhibitors and risk of a fatal or severe course of illness in patients with COVID-19
Chia Siang Kow, Syed Shahzad Hasan
Therapies.2021; 76(4): 361. CrossRef - Disentangling conflicting evidence on DPP-4 inhibitors and outcomes of COVID-19: narrative review and meta-analysis
B. M. Bonora, A. Avogaro, G. P. Fadini
Journal of Endocrinological Investigation.2021; 44(7): 1379. CrossRef - Prognostic bioindicators in severe COVID-19 patients
L. Bergantini, E. Bargagli, M. d'Alessandro, R.M. Refini, P. Cameli, L. Galasso, C. Scapellato, F. Montagnani, S. Scolletta, F. Franchi, S. Valente, D. Bennett, G. Sebastiani, B. Frediani, F. Dotta
Cytokine.2021; 141: 155455. CrossRef - Epidemiological characteristics and outcomes of COVID-19 in diabetic versus non-diabetic patients
Leila Moftakhar, Parisa Moftakhar, Elahe Piraee, Haleh Ghaem, Aliasghar Valipour, Habibollah Azarbakhsh
International Journal of Diabetes in Developing Countries.2021; 41(3): 383. CrossRef - DPP-4 inhibition and COVID-19: From initial concerns to recent expectations
André J. Scheen
Diabetes & Metabolism.2021; 47(2): 101213. CrossRef - Use of dipeptidyl peptidase‐4 inhibitors and prognosis of COVID‐19 in hospitalized patients with type 2 diabetes: A propensity score analysis from the CORONADO study
Ronan Roussel, Patrice Darmon, Matthieu Pichelin, Thomas Goronflot, Yawa Abouleka, Leila Ait Bachir, Ingrid Allix, Deborah Ancelle, Sara Barraud, Lyse Bordier, Aurélie Carlier, Nicolas Chevalier, Christine Coffin‐Boutreux, Emmanuel Cosson, Anne Dorange, O
Diabetes, Obesity and Metabolism.2021; 23(5): 1162. CrossRef - Dipeptidyl peptidase-4 inhibitor use and mortality in COVID-19 patients with diabetes mellitus: an updated systematic review and meta-analysis
Rimesh Pal, Mainak Banerjee, Soham Mukherjee, Ranjitpal Singh Bhogal, Amanpreet Kaur, Sanjay K. Bhadada
Therapeutic Advances in Endocrinology and Metabolism.2021; 12: 204201882199648. CrossRef - Renin–angiotensin-system inhibitors and all-cause mortality in patients with COVID-19: a systematic review and meta-analysis of observational studies
Chirag Bavishi, Paul K. Whelton, Giuseppe Mancia, Giovanni Corrao, Franz H. Messerli
Journal of Hypertension.2021; 39(4): 784. CrossRef - Evaluation of the Current Therapeutic Approaches for COVID-19: A Systematic Review and a Meta-analysis
Zeinab Abdelrahman, Qian Liu, Shanmei Jiang, Mengyuan Li, Qingrong Sun, Yue Zhang, Xiaosheng Wang
Frontiers in Pharmacology.2021;[Epub] CrossRef - Dipeptidyl peptidase 4 (DPP4) inhibitor and outcome from coronavirus disease 2019 (COVID-19) in diabetic patients: a systematic review, meta-analysis, and meta-regression
Timotius Ivan Hariyanto, Andree Kurniawan
Journal of Diabetes & Metabolic Disorders.2021; 20(1): 543. CrossRef - Impact of diabetes mellitus on in-hospital mortality in adult patients with COVID-19: a systematic review and meta-analysis
Halla Kaminska, Lukasz Szarpak, Dariusz Kosior, Wojciech Wieczorek, Agnieszka Szarpak, Mahdi Al-Jeabory, Wladyslaw Gawel, Aleksandra Gasecka, Milosz J. Jaguszewski, Przemyslawa Jarosz-Chobot
Acta Diabetologica.2021; 58(8): 1101. CrossRef - Dipeptidyl peptidase-4 (DPP-4) inhibitor and mortality in coronavirus disease 2019 (COVID-19) – A systematic review, meta-analysis, and meta-regression
Iis Inayati Rakhmat, Yudith Yunia Kusmala, Dewi Ratih Handayani, Henny Juliastuti, Eka Noneng Nawangsih, Arief Wibowo, Michael Anthonius Lim, Raymond Pranata
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2021; 15(3): 777. CrossRef - Post-infection depressive, anxiety and post-traumatic stress symptoms: A prospective cohort study in patients with mild COVID-19
Flavia Ismael, João C.S. Bizario, Tatiane Battagin, Beatriz Zaramella, Fabio E. Leal, Julio Torales, Antonio Ventriglio, Megan E. Marziali, Silvia S. Martins, João M. Castaldelli-Maia
Progress in Neuro-Psychopharmacology and Biological Psychiatry.2021; 111: 110341. CrossRef - Managing diabetes in diabetic patients with COVID: where do we start from?
Angelo Avogaro, Benedetta Bonora, Gian Paolo Fadini
Acta Diabetologica.2021; 58(11): 1441. CrossRef - Is diabetes mellitus a wrongdoer to COVID-19 severity?
Sanjib Sarkar, Dibyendu Das, Sawlang Borsingh Wann, Jatin Kalita, Prasenjit Manna
Diabetes Research and Clinical Practice.2021; 178: 108936. CrossRef - Dipeptidyl Peptidase 4 Inhibitor, an Update
Ju Hee Lee
The Journal of Korean Diabetes.2021; 22(2): 91. CrossRef - Correlation Analysis Between Serum Uric Acid, Prealbumin Level, Lactate Dehydrogenase, and Severity of COVID-19
Zhenmu Jin, Mo Zheng, Jichan Shi, Xinchun Ye, Fang Cheng, Que-Lu Chen, Jianping Huang, Xian-Gao Jiang
Frontiers in Molecular Biosciences.2021;[Epub] CrossRef - Association Between Glucagon-Like Peptide 1 Receptor Agonist and Sodium–Glucose Cotransporter 2 Inhibitor Use and COVID-19 Outcomes
Anna R. Kahkoska, Trine Julie Abrahamsen, G. Caleb Alexander, Tellen D. Bennett, Christopher G. Chute, Melissa A. Haendel, Klara R. Klein, Hemalkumar Mehta, Joshua D. Miller, Richard A. Moffitt, Til Stürmer, Kajsa Kvist, John B. Buse, Tim Q. Duong
Diabetes Care.2021; 44(7): 1564. CrossRef - The effect of metformin on mortality and severity in COVID-19 patients with diabetes mellitus
Wenxing Yang, Xuehong Sun, Jun Zhang, Kui Zhang
Diabetes Research and Clinical Practice.2021; 178: 108977. CrossRef - Renin‐Angiotensin Aldosterone System Inhibitors in Primary Prevention and COVID‐19
Jordan Loader, Erik Lampa, Stefan Gustafsson, Thomas Cars, Johan Sundström
Journal of the American Heart Association.2021;[Epub] CrossRef - Factors influencing on development of COVID-19 pneumonia and association with oral anti-diabetic drugs in hospitalized patients with diabetes mellitus
Ayça Elibol, Didem Eren, Macide Deniz Erdoğan, Merve Elmaağaç, Oguzhan Sıtkı Dizdar, İlhami Çelik, Ali İhsan Günal
Primary Care Diabetes.2021; 15(5): 806. CrossRef - Aging & COVID-19 susceptibility, disease severity, and clinical outcomes: The role of entangled risk factors
Melina Farshbafnadi, Sara Kamali Zonouzi, Mohammadmahdi Sabahi, Mahsa Dolatshahi, Mohammad Hadi Aarabi
Experimental Gerontology.2021; 154: 111507. CrossRef - Classical and Counter-Regulatory Renin–Angiotensin System: Potential Key Roles in COVID-19 Pathophysiology
Moudhi Almutlaq, Abir Abdullah Alamro, Fayhan Alroqi, Tlili Barhoumi
CJC Open.2021; 3(8): 1060. CrossRef - Metformin in Patients With COVID-19: A Systematic Review and Meta-Analysis
Yin Li, Xue Yang, Peijing Yan, Tong Sun, Zhi Zeng, Sheyu Li
Frontiers in Medicine.2021;[Epub] CrossRef - Pre-existing health conditions and severe COVID-19 outcomes: an umbrella review approach and meta-analysis of global evidence
Marina Treskova-Schwarzbach, Laura Haas, Sarah Reda, Antonia Pilic, Anna Borodova, Kasra Karimi, Judith Koch, Teresa Nygren, Stefan Scholz, Viktoria Schönfeld, Sabine Vygen-Bonnet, Ole Wichmann, Thomas Harder
BMC Medicine.2021;[Epub] CrossRef - COVID-19 Vaccination for Endocrine Patients: A Position Statement from the Korean Endocrine Society
Cheol Ryong Ku, Kyong Yeun Jung, Chang Ho Ahn, Jun Sung Moon, Ju Hee Lee, Eun Heui Kim, Hyemi Kwon, Hee Kyung Kim, Sunghwan Suh, Sangmo Hong, Jeonghoon Ha, Eun Roh, Jin Hwa Kim, Mi-kyung Kim
Endocrinology and Metabolism.2021; 36(4): 757. CrossRef - High Fibrosis-4 Index Is Related with Worse Clinical Outcome in Patients with Coronavirus Disease 2019 and Diabetes Mellitus: A Multicenter Observational Study
Sung-Woo Kim, Jae-Han Jeon, Jun Sung Moon, Mi Kyung Kim
Endocrinology and Metabolism.2021; 36(4): 800. CrossRef - Mortality Risk of Antidiabetic Agents for Type 2 Diabetes With COVID-19: A Systematic Review and Meta-Analysis
Chengxia Kan, Yang Zhang, Fang Han, Qian Xu, Tongtong Ye, Ningning Hou, Xiaodong Sun
Frontiers in Endocrinology.2021;[Epub] CrossRef - Analysis of influence of background therapy for comorbidities in the period before infection on the risk of the lethal COVID outcome. Data from the international ACTIV SARS-CoV-2 registry («Analysis of chronic non-infectious diseases dynamics after COVID-
E. I. Tarlovskaya, A. G. Arutyunov, A. O. Konradi, Yu. M. Lopatin, A. P. Rebrov, S. N. Tereshchenko, A. I. Chesnikova, H. G. Hayrapetyan, A. P. Babin, I. G. Bakulin, N. V. Bakulina, L. A. Balykova, A. S. Blagonravova, M. V. Boldina, A. R. Vaisberg, A. S.
Kardiologiia.2021; 61(9): 20. CrossRef - Association of clinical characteristics, antidiabetic and cardiovascular agents with diabetes mellitus and COVID-19: a 7-month follow-up cohort study
Marzieh Pazoki, Fatemeh Chichagi, Azar Hadadi, Samira Kafan, Mahnaz Montazeri, Sina Kazemian, Arya Aminorroaya, Mehdi Ebrahimi, Haleh Ashraf, Mojgan Mirabdolhagh Hazaveh, Mohammad Reza Khajavi, Reza Shariat Moharari, Seyed Hamidreza Sharifnia, Shahrokh Ka
Journal of Diabetes & Metabolic Disorders.2021; 20(2): 1545. CrossRef - COVID-19 and Diabetes: A Comprehensive Review of Angiotensin Converting Enzyme 2, Mutual Effects and Pharmacotherapy
Lingli Xie, Ziying Zhang, Qian Wang, Yangwen Chen, Dexue Lu, Weihua Wu
Frontiers in Endocrinology.2021;[Epub] CrossRef - Impact of Diabetes on COVID-19 Mortality and Hospital Outcomes, a Global Perspective: An ONTOP Systematic Review and Meta-Analysis
Stavroula Kastora, Manisha Patel, Ben Carter, Mirela Delibegovic, Phyo Kyaw Myint
SSRN Electronic Journal .2021;[Epub] CrossRef - Decision Trees: Predictions of Global Vulnerability to Coronavirus Outbreaks
Moacir José da Silva
SSRN Electronic Journal .2020;[Epub] CrossRef - The potential association between common comorbidities and severity and mortality of coronavirus disease 2019: A pooled analysis
Liman Luo, Menglu Fu, Yuanyuan Li, Shuiqing Hu, Jinlan Luo, Zhihui Chen, Jing Yu, Wenhua Li, Ruolan Dong, Yan Yang, Ling Tu, Xizhen Xu
Clinical Cardiology.2020; 43(12): 1478. CrossRef - The Effect of Metformin Consumption on Mortality in Hospitalized COVID-19 patients: a systematic review and meta-analysis
Antonia Anna Lukito, Raymond Pranata, Joshua Henrina, Michael Anthonius Lim, Sherly Lawrensia, Ketut Suastika
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2020; 14(6): 2177. CrossRef - Risk Factors on the Progression to Clinical Outcomes of COVID-19 Patients in South Korea: Using National Data
Seon-Rye Kim, Seoul-Hee Nam, Yu-Rin Kim
International Journal of Environmental Research and Public Health.2020; 17(23): 8847. CrossRef - Clinical Outcomes of COVID-19 Patients with Type 2 Diabetes: A Population-Based Study in Korea
Ji Hong You, Sang Ah Lee, Sung-Youn Chun, Sun Ok Song, Byung-Wan Lee, Dae Jung Kim, Edward J. Boyko
Endocrinology and Metabolism.2020; 35(4): 901. CrossRef
- Potential use of sodium glucose co-transporter 2 inhibitors during acute illness: a systematic review based on COVID-19
- Letter: Presence of Carotid Plaque Is Associated with Rapid Renal Function Decline in Patients with Type 2 Diabetes Mellitus and Normal Renal Function (
Diabetes Metab J 2019;43:840–53) - Min-Ji Kim, Jae-Han Jeon
- Diabetes Metab J. 2020;44(1):201-202. Published online February 21, 2020
- DOI: https://doi.org/10.4093/dmj.2020.0017
- 3,302 View
- 43 Download
- Epidemiology
- Low-Normal Free Thyroxine Levels in Euthyroid Male Are Associated with Prediabetes
- Sung Woo Kim, Jae-Han Jeon, Jun Sung Moon, Eon Ju Jeon, Mi-Kyung Kim, In-Kyu Lee, Jung Beom Seo, Keun-Gyu Park
- Diabetes Metab J. 2019;43(5):718-726. Published online March 19, 2019
- DOI: https://doi.org/10.4093/dmj.2018.0222
- 4,320 View
- 51 Download
- 2 Web of Science
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader Abnormal thyroid function is associated with impaired glucose homeostasis. This study aimed to determine whether free thyroxine (FT4) influences the prevalence of prediabetes in euthyroid subjects using a cross-sectional survey derived from the Korea National Health and Nutrition Examination Survey, conducted between 2013 and 2015. We studied 2,399 male participants of >20 years of age who were euthyroid and non-diabetic. Prediabetic participants had lower FT4 concentrations than those without prediabetes, but their thyrotropin concentrations were similar. We stratified the population into tertiles according to FT4 concentration. After adjusting for multiple confounding factors, glycosylated hemoglobin (HbA1c) levels significantly decreased with increasing FT4 tertile, whereas fasting plasma glucose (FPG) levels were not associated with FT4 tertiles (HbA1c,
P <0.01 in T3 vs. T1; FPG,P =0.489 in T3 vs. T1). The prevalence of prediabetes was significantly higher in T1 (odds ratio, 1.426; 95% confidence interval, 1.126 to 1.806;P <0.01) than in T3. In conclusion, subjects with low-normal serum FT4 had high HbA1c and were more likely to have prediabetes. These results suggest that low FT4 concentration is a risk factor for prediabetes in male, even when thyroid function is within the normal range.
- Islet Studies and Transplantation
-
- Myricetin Protects Against High Glucose-Induced β-Cell Apoptosis by Attenuating Endoplasmic Reticulum Stress via Inactivation of Cyclin-Dependent Kinase 5
- Udayakumar Karunakaran, Suma Elumalai, Jun Sung Moon, Jae-Han Jeon, Nam Doo Kim, Keun-Gyu Park, Kyu Chang Won, Jaechan Leem, In-Kyu Lee
- Diabetes Metab J. 2019;43(2):192-205. Published online January 16, 2019
- DOI: https://doi.org/10.4093/dmj.2018.0052
- 4,903 View
- 106 Download
- 33 Web of Science
- 32 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader Background Chronic hyperglycemia has deleterious effects on pancreatic β-cell function and turnover. Recent studies support the view that cyclin-dependent kinase 5 (CDK5) plays a role in β-cell failure under hyperglycemic conditions. However, little is known about how CDK5 impair β-cell function. Myricetin, a natural flavonoid, has therapeutic potential for the treatment of type 2 diabetes mellitus. In this study, we examined the effect of myricetin on high glucose (HG)-induced β-cell apoptosis and explored the relationship between myricetin and CDK5.
Methods To address this question, we subjected INS-1 cells and isolated rat islets to HG conditions (30 mM) in the presence or absence of myricetin. Docking studies were conducted to validate the interaction between myricetin and CDK5. Gene expression and protein levels of endoplasmic reticulum (ER) stress markers were measured by real-time reverse transcription polymerase chain reaction and Western blot analysis.
Results Activation of CDK5 in response to HG coupled with the induction of ER stress via the down regulation of sarcoendoplasmic reticulum calcium ATPase 2b (
SERCA2b ) gene expression and reduced the nuclear accumulation of pancreatic duodenal homeobox 1 (PDX1) leads to β-cell apoptosis. Docking study predicts that myricetin inhibit CDK5 activation by direct binding in the ATP-binding pocket. Myricetin counteracted the decrease in the levels of PDX1 and SERCA2b by HG. Moreover, myricetin attenuated HG-induced apoptosis in INS-1 cells and rat islets and reduce the mitochondrial dysfunction by decreasing reactive oxygen species production and mitochondrial membrane potential (Δψm) loss.Conclusion Myricetin protects the β-cells against HG-induced apoptosis by inhibiting ER stress, possibly through inactivation of CDK5 and consequent upregulation of PDX1 and SERCA2b.
-
Citations
Citations to this article as recorded by- Profiling of secondary metabolite and evaluation of anti-diabetic potency of Crotalaria quinquefolia (L): In-vitro, in-vivo, and in-silico approaches
Nazmun Nahar, Md. Nazmul Hasan Zilani, Partha Biswas, Md. Morsaline Billah, Shabana Bibi, Norah A. Albekairi, Abdulrahman Alshammari, Md. Nazmul Hasan
Saudi Pharmaceutical Journal.2024; 32(1): 101887. CrossRef - Mitochondrial aldehyde dehydrogenase-2 coordinates the hydrogen sulfide - AMPK axis to attenuate high glucose-induced pancreatic β-cell dysfunction by glutathione antioxidant system
Udayakumar Karunakaran, Suma Elumalai, Seung Min Chung, Kathrin Maedler, Kyu Chang Won, Jun Sung Moon
Redox Biology.2024; 69: 102994. CrossRef - Network-based identification and mechanism exploration of active ingredients against Alzheimer’s disease via targeting endoplasmic reticulum stress from traditional chinese medicine
Zhao Dai, Tian Hu, Junwen Wei, Xue Wang, Chuipu Cai, Yong Gu, Yunhui Hu, Wenjia Wang, Qihui Wu, Jiansong Fang
Computational and Structural Biotechnology Journal.2024; 23: 506. CrossRef - Myricetin as a Promising Flavonoid with Multitargeted Biological Activity
A.S. Chiriapkin
Juvenis Scientia.2024; 10(1): 5. CrossRef - Naturally occurring small molecules with dual effect upon inflammatory signaling pathways and endoplasmic reticulum stress response
Daniela Correia da Silva, Patrícia Valentão, David M. Pereira
Journal of Physiology and Biochemistry.2024;[Epub] CrossRef - Omnifarious fruit polyphenols: an omnipotent strategy to prevent and intervene diabetes and related complication?
Yao Chen, Xuejiao Qie, Wei Quan, Maomao Zeng, Fang Qin, Jie Chen, Benu Adhikari, Zhiyong He
Critical Reviews in Food Science and Nutrition.2023; 63(20): 4288. CrossRef - Regulation of reactive oxygen species by phytochemicals for the management of cancer and diabetes
Heui Min Lim, See-Hyoung Park
Critical Reviews in Food Science and Nutrition.2023; 63(22): 5911. CrossRef - Bioactive compounds from Polygonatum genus as anti-diabetic agents with future perspectives
Yan Shi, Dun Si, Donghong Chen, Xinfeng Zhang, Zhigang Han, Qiang Yu, Jingjing Liu, Jinping Si
Food Chemistry.2023; 408: 135183. CrossRef - Venom Peptides, Polyphenols and Alkaloids: Are They the Next Antidiabetics That Will Preserve β-Cell Mass and Function in Type 2 Diabetes?
Michele Lodato, Valérie Plaisance, Valérie Pawlowski, Maxime Kwapich, Alexandre Barras, Emeline Buissart, Stéphane Dalle, Sabine Szunerits, Jérôme Vicogne, Rabah Boukherroub, Amar Abderrahmani
Cells.2023; 12(6): 940. CrossRef - TFP5 attenuates cyclin‐dependent kinase 5‐mediated islet β‐cell damage in diabetes
Shunyao Liu, Bo Li, Danna Ma, Yuejia Tao, Jiang Song, Li Bao, Guoqing Zhang, Hongyan Luo, Shilu Cao, Jing E, Yali Zheng
Chemical Biology & Drug Design.2023; 102(1): 76. CrossRef - Antiviral and Possible Prophylactic Significance of Myricetin for COVID-19
Pawan K. Agrawal, Chandan Agrawal, Gerald Blunden
Natural Product Communications.2023; 18(4): 1934578X2311662. CrossRef - In Vitro and In Silico Protocols for the Assessment of Anti-Tick Compounds from Pinus roxburghii against Rhipicephalus (Boophilus) microplus Ticks
Sana Ayub, Nosheen Malak, Raquel Cossío-Bayúgar, Nasreen Nasreen, Afshan Khan, Sadaf Niaz, Adil Khan, Abdallah D. Alanazi, Mourad Ben Said
Animals.2023; 13(8): 1388. CrossRef - Protective Effect of Myricetin Against Experimentally Induced Torsion in Rats
M. Tatar, Z. Polat, J. Öner, H. Öner
Biology Bulletin.2023; 50(6): 1338. CrossRef - The pharmacological mechanism of Abelmoschus manihot in the treatment of chronic kidney disease
Cuiting Wei, Chao Wang, Run Li, Yunfeng Bai, Xue Wang, Qingyun Fang, Xiangmei Chen, Ping Li
Heliyon.2023; 9(11): e22017. CrossRef - Natural Polyphenols as SERCA Activators: Role in the Endoplasmic Reticulum Stress-Related Diseases
Jana Viskupicova, Petronela Rezbarikova
Molecules.2022; 27(16): 5095. CrossRef - Research progress on the mechanism of beta-cell apoptosis in type 2 diabetes mellitus
SuFang You, JingYi Zheng, YuPing Chen, HuiBin Huang
Frontiers in Endocrinology.2022;[Epub] CrossRef - Myricetin inhibits pseudorabies virus infection through direct inactivation and activating host antiviral defense
Huaiyue Hu, Zhiqiang Hu, Yingying Zhang, Hongping Wan, Zhongqiong Yin, Lixia Li, Xiaoxia Liang, Xinghong Zhao, Lizi Yin, Gang Ye, Yuan-Feng Zou, Huaqiao Tang, Renyong Jia, Yaqin Chen, Hao Zhou, Xu Song
Frontiers in Microbiology.2022;[Epub] CrossRef - Effects of myricetin against cadmium-induced neurotoxicity in PC12 cells
Azadeh Aminzadeh, Ayda Salarinejad
Toxicology Research.2021; 10(1): 84. CrossRef - Pioglitazone-induced AMPK-Glutaminase-1 prevents high glucose-induced pancreatic β-cell dysfunction by glutathione antioxidant system
Udayakumar Karunakaran, Suma Elumalai, Jun Sung Moon, Kyu Chang Won
Redox Biology.2021; 45: 102029. CrossRef - Chlorogenic acid and β-glucan from highland barley grain ameliorate β-cell dysfunction via inhibiting apoptosis and improving cell proliferation
Ze-Hua Liu, Bo Li
Food & Function.2021; 12(20): 10040. CrossRef - The cyclin dependent kinase inhibitor Roscovitine prevents diet-induced metabolic disruption in obese mice
Nabil Rabhi, Kathleen Desevin, Briana Noel Cortez, Ryan Hekman, Jean Z. Lin, Andrew Emili, Stephen R. Farmer
Scientific Reports.2021;[Epub] CrossRef - AdipoRon promotes diabetic fracture repair through endochondral ossification-based bone repair by enhancing survival and differentiation of chondrocytes
Zhongyi Wang, Jinxin Tang, Ying Li, Yu Wang, Yanyang Guo, Qisheng Tu, Jake Chen, Chen Wang
Experimental Cell Research.2020; 387(2): 111757. CrossRef - A kinase of many talents: non-neuronal functions of CDK5 in development and disease
Samanta Sharma, Piotr Sicinski
Open Biology.2020; 10(1): 190287. CrossRef - Mitochondrial dysfunction in the fetoplacental unit in gestational diabetes mellitus
Luis Sobrevia, Paola Valero, Adriana Grismaldo, Roberto Villalobos-Labra, Fabián Pardo, Mario Subiabre, Gael Armstrong, Fernando Toledo, Sofía Vega, Marcelo Cornejo, Gonzalo Fuentes, Reinaldo Marín
Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease.2020; 1866(12): 165948. CrossRef - Myricetin bioactive effects: moving from preclinical evidence to potential clinical applications
Yasaman Taheri, Hafiz Ansar Rasul Suleria, Natália Martins, Oksana Sytar, Ahmet Beyatli, Balakyz Yeskaliyeva, Gulnaz Seitimova, Bahare Salehi, Prabhakar Semwal, Sakshi Painuli, Anuj Kumar, Elena Azzini, Miquel Martorell, William N. Setzer, Alfred Maroyi,
BMC Complementary Medicine and Therapies.2020;[Epub] CrossRef - Current Pharmacological Trends on Myricetin
Gudiya Gupta, Mohd Aftab Siddiqui, Mohd Muazzam Khan, Mohd Ajmal, Rabiya Ahsan, Md Azizur Rahaman, Md Afroz Ahmad, Md Arshad, Mohammad Khushtar
Drug Research.2020;[Epub] CrossRef - Silencing cyclophilin A improves insulin secretion, reduces cell apoptosis, and alleviates inflammation as well as oxidant stress in high glucose-induced pancreatic β-cells via MAPK/NF-kb signaling pathway
Tangying Li, Huibiao Quan, Huachuan Zhang, Leweihua Lin, Qianying Ou, Kaining Chen
Bioengineered.2020; 11(1): 1047. CrossRef - Endoplasmic reticulum stress contributes to NMDA-induced pancreatic β-cell dysfunction in a CHOP-dependent manner
Xiao-Ting Huang, Wei Liu, Yong Zhou, Mei Sun, Chen-Chen Sun, Chen-Yu Zhang, Si-Yuan Tang
Life Sciences.2019; 232: 116612. CrossRef - Dapagliflozin rescues endoplasmic reticulum stress-mediated cell death
Ryo Shibusawa, Eijiro Yamada, Shuichi Okada, Yasuyo Nakajima, Claire C. Bastie, Akito Maeshima, Kyoichi Kaira, Masanobu Yamada
Scientific Reports.2019;[Epub] CrossRef - Myricetin prevents thapsigargin-induced CDK5-P66Shc signalosome mediated pancreatic β-cell dysfunction
Udayakumar Karunakaran, Ji Eun Lee, Suma Elumalai, Jun Sung Moon, Kyu Chang Won
Free Radical Biology and Medicine.2019; 141: 59. CrossRef - CDK5: Key Regulator of Apoptosis and Cell Survival
Rabih Roufayel, Nimer Murshid
Biomedicines.2019; 7(4): 88. CrossRef - Oral DhHP-6 for the Treatment of Type 2 Diabetes Mellitus
Kai Wang, Yu Su, Yuting Liang, Yanhui Song, Liping Wang
International Journal of Molecular Sciences.2019; 20(6): 1517. CrossRef
- Profiling of secondary metabolite and evaluation of anti-diabetic potency of Crotalaria quinquefolia (L): In-vitro, in-vivo, and in-silico approaches
- Obesity and Metabolic Syndrome
- Role of the Pyruvate Dehydrogenase Complex in Metabolic Remodeling: Differential Pyruvate Dehydrogenase Complex Functions in Metabolism
- Sungmi Park, Jae-Han Jeon, Byong-Keol Min, Chae-Myeong Ha, Themis Thoudam, Bo-Yoon Park, In-Kyu Lee
- Diabetes Metab J. 2018;42(4):270-281. Published online August 21, 2018
- DOI: https://doi.org/10.4093/dmj.2018.0101
- 9,955 View
- 299 Download
- 102 Web of Science
- 102 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Mitochondrial dysfunction is a hallmark of metabolic diseases such as obesity, type 2 diabetes mellitus, neurodegenerative diseases, and cancers. Dysfunction occurs in part because of altered regulation of the mitochondrial pyruvate dehydrogenase complex (PDC), which acts as a central metabolic node that mediates pyruvate oxidation after glycolysis and fuels the Krebs cycle to meet energy demands. Fine-tuning of PDC activity has been mainly attributed to post-translational modifications of its subunits, including the extensively studied phosphorylation and de-phosphorylation of the E1α subunit of pyruvate dehydrogenase (PDH), modulated by kinases (pyruvate dehydrogenase kinase [PDK] 1-4) and phosphatases (pyruvate dehydrogenase phosphatase [PDP] 1-2), respectively. In addition to phosphorylation, other covalent modifications, including acetylation and succinylation, and changes in metabolite levels via metabolic pathways linked to utilization of glucose, fatty acids, and amino acids, have been identified. In this review, we will summarize the roles of PDC in diverse tissues and how regulation of its activity is affected in various metabolic disorders.
-
Citations
Citations to this article as recorded by- Mitochondria dysfunction induced by decyl-TPP mitochondriotropic antioxidant based on caffeic acid AntiOxCIN6 sensitizes cisplatin lung anticancer therapy due to a remodeling of energy metabolism
Ricardo Amorim, Carina C. Magalhães, Sofia Benfeito, Fernando Cagide, Ludgero C. Tavares, Katia Santos, Vilma A. Sardão, Sandipan Datta, Gino A. Cortopassi, Inês Baldeiras, John G. Jones, Fernanda Borges, Paulo J. Oliveira, José Teixeira
Biochemical Pharmacology.2024; 219: 115953. CrossRef - Thiamine analogues featuring amino-oxetanes as potent and selective inhibitors of pyruvate dehydrogenase
Alex H.Y. Chan, Terence C.S. Ho, Finian J. Leeper
Bioorganic & Medicinal Chemistry Letters.2024; 98: 129571. CrossRef - Pyruvate Dehydrogenase Complex in Neonatal Hypoxic–Ischemic Brain Injury
Tao Zhou, Yuangao Zhong, Yong Zhang, Yue Zhou
ACS Pharmacology & Translational Science.2024; 7(1): 42. CrossRef - Biomarkers of heart failure: advances in omics studies
Kuo Chi, Jing Liu, Xinghua Li, He Wang, Yanliang Li, Qingnan Liu, Yabin Zhou, Yuan Ge
Molecular Omics.2024; 20(3): 169. CrossRef - Effects of ambient UVB light on Pacific oyster Crassostrea gigas mantle tissue based on multivariate data
Hongce Song, Chaoyi Xie, Meiyun Dong, Yuxuan Zhang, Haifeng Huang, Yijing Han, Yaqiong Liu, Lei Wei, Xiaotong Wang
Ecotoxicology and Environmental Safety.2024; 274: 116236. CrossRef - Autocrine phosphatase PDP2 inhibits ferroptosis by dephosphorylating ACSL4 in the Luminal A Breast Cancer
Jun-Jie Zhu, Feng-Ying Huang, Hengyu Chen, Yun-long Zhang, Ming-Hui Chen, Ri-Hong Wu, Shu-Zhen Dai, Gui-Sheng He, Guang-Hong Tan, Wu-Ping Zheng, Sreeparna Banerjee
PLOS ONE.2024; 19(3): e0299571. CrossRef - Glutaminase potentiates the glycolysis in esophageal squamous cell carcinoma by interacting with PDK1
Guangzhao Zhu, Fangxia Guan, Shenglei Li, Qing Zhang, Xueying Zhang, Yue Qin, Zhangzhan Sun, Shaohua Peng, Jiexing Cheng, Yiyang Li, Ruili Ren, Tianli Fan, Hongtao Liu
Molecular Carcinogenesis.2024; 63(5): 897. CrossRef - In Vitro and In Silico Studies on Cytotoxic Properties of Oxythiamine and 2′-Methylthiamine
Marta Malinowska, Magdalena Czerniecka, Izabella Jastrzebska, Artur Ratkiewicz, Adam Tylicki, Natalia Wawrusiewicz-Kurylonek
International Journal of Molecular Sciences.2024; 25(8): 4359. CrossRef - Inhibition of Pyruvate Dehydrogenase Kinase 4 in CD4+ T Cells Ameliorates Intestinal Inflammation
Hoyul Lee, Jae Han Jeon, Yu-Jeong Lee, Mi-Jin Kim, Woong Hee Kwon, Dipanjan Chanda, Themis Thoudam, Haushabhau S. Pagire, Suvarna H. Pagire, Jin Hee Ahn, Robert A. Harris, Eun Soo Kim, In-Kyu Lee
Cellular and Molecular Gastroenterology and Hepatology.2023; 15(2): 439. CrossRef - Nitric oxide regulation of cellular metabolism: Adaptive tuning of cellular energy
Gregory Pappas, Melissa L. Wilkinson, Andrew J. Gow
Nitric Oxide.2023; 131: 8. CrossRef - Furan-based inhibitors of pyruvate dehydrogenase: SAR study, biochemical evaluation and computational analysis
Alex H. Y. Chan, Terence C. S. Ho, Daniel R. Parle, Finian J. Leeper
Organic & Biomolecular Chemistry.2023; 21(8): 1755. CrossRef - The pyruvate dehydrogenase complex: Life’s essential, vulnerable and druggable energy homeostat
Peter W. Stacpoole, Charles E. McCall
Mitochondrion.2023; 70: 59. CrossRef - The Impact of Krebs Cycle Intermediates on the Endocrine System and Immune System: A Comparison
Borros M. Arneth
Endocrines.2023; 4(1): 179. CrossRef - Consequences of reprogramming acetyl-CoA metabolism by 2,3,7,8-tetrachlorodibenzo-p-dioxin in the mouse liver
Giovan N. Cholico, Karina Orlowska, Russell R. Fling, Warren J. Sink, Nicholas A. Zacharewski, Kelly A. Fader, Rance Nault, Tim Zacharewski
Scientific Reports.2023;[Epub] CrossRef - The Link between Mitochondrial Dysfunction and Sarcopenia: An Update Focusing on the Role of Pyruvate Dehydrogenase Kinase 4
Min-Ji Kim, Ibotombi Singh Sinam, Zerwa Siddique, Jae-Han Jeon, In-Kyu Lee
Diabetes & Metabolism Journal.2023; 47(2): 153. CrossRef - Targeting PDK2 rescues stress-induced impaired brain energy metabolism
Changshui Wang, Changmeng Cui, Pengfei Xu, Li Zhu, Hongjia Xue, Beibei Chen, Pei Jiang
Molecular Psychiatry.2023; 28(10): 4138. CrossRef - The Role of Pyruvate Metabolism in Mitochondrial Quality Control and Inflammation
Min-Ji Kim, Hoyul Lee, Dipanjan Chanda, Themis Thoudam, Hyeon-Ji Kang, Robert A. Harris, In-Kyu Lee
Molecules and Cells.2023; 46(5): 259. CrossRef - Inhibition of pyruvate dehydrogenase kinase 4 ameliorates kidney ischemia-reperfusion injury by reducing succinate accumulation during ischemia and preserving mitochondrial function during reperfusion
Chang Joo Oh, Min-Ji Kim, Ji-Min Lee, Dong Hun Kim, Il-Young Kim, Sanghee Park, Yeongmin Kim, Kyung-Bok Lee, Sang-Hee Lee, Chae Won Lim, Myeongjin Kim, Jung-Yi Lee, Haushabhau S. Pagire, Suvarna H. Pagire, Myung Ae Bae, Dipanjan Chanda, Themis Thoudam, Ah
Kidney International.2023; 104(4): 724. CrossRef - PDHA1 hyperacetylation-mediated lactate overproduction promotes sepsis-induced acute kidney injury via Fis1 lactylation
Sheng An, Yi Yao, Hongbin Hu, Junjie Wu, Jiaxin Li, Lulan Li, Jie Wu, Maomao Sun, Zhiya Deng, Yaoyuan Zhang, Shenhai Gong, Qiaobing Huang, Zhongqing Chen, Zhenhua Zeng
Cell Death & Disease.2023;[Epub] CrossRef - Iron promotes glycolysis to drive colon tumorigenesis
Zhaoli Liu, Luke Villareal, Lavanya Goodla, Hyeoncheol Kim, Daniel M. Falcon, Mohammad Haneef, David R. Martin, Li Zhang, Ho-Joon Lee, Daniel Kremer, Costas A. Lyssiotis, Yatrik M. Shah, Henry C. Lin, Hui-kuan Lin, Xiang Xue
Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease.2023; 1869(8): 166846. CrossRef - Driving force of deteriorated cellular environment in heart failure: Metabolic remodeling
Lu Fan, Chenchen Meng, Xiaoming Wang, Yunjiao Wang, Yanyang Li, Shichao Lv, Junping Zhang
Clinics.2023; 78: 100263. CrossRef - Mitochondrial dysfunctions in T cells: focus on inflammatory bowel disease
Hoyul Lee, Jae-Han Jeon, Eun Soo Kim
Frontiers in Immunology.2023;[Epub] CrossRef - Adrenomedullin induces cisplatin chemoresistance in ovarian cancer through reprogramming of glucose metabolism
Lei Dou, Enting Lu, Dongli Tian, Fangmei Li, Lei Deng, Yi Zhang
Journal of Translational Internal Medicine.2023; 11(2): 169. CrossRef - Metabolic deregulation associated with aging modulates protein aggregation in the yeast model of Huntington’s disease
Sai Sanwid Pradhan, Sai Swaroop R., Sai Phalguna Kanikaram, Datta Darshan V.M., Ashish Pargaonkar, Rajesh Babu Dandamudi, Venketesh Sivaramakrishnan
Journal of Biomolecular Structure and Dynamics.2023; : 1. CrossRef - Unique and generic crossed metabolism in response to four sub-lethal environmental stresses in the oriental fruit fly, Bactrocera dorsalis Hendel
Lili Ren, Hongxia Zhang, Jiao Zhou, Yajing Wu, Bo Liu, Shuping Wang, Xin Liu, Xin Hao, Lilin Zhao
Ecotoxicology and Environmental Safety.2023; 264: 115434. CrossRef - Oxidative stress regulation and related metabolic pathways in epithelial–mesenchymal transition of breast cancer stem cells
Raheleh Farahzadi, Behnaz Valipour, Ezzatollah Fathi, Samaneh Pirmoradi, Ommoleila Molavi, Soheila Montazersaheb, Zohreh Sanaat
Stem Cell Research & Therapy.2023;[Epub] CrossRef - Preclinical Study in Mouse Thymus and Thymocytes: Effects of Treatment with a Combination of Sodium Dichloroacetate and Sodium Valproate on Infectious Inflammation Pathways
Donatas Stakišaitis, Linas Kapočius, Evelina Kilimaitė, Dovydas Gečys, Lina Šlekienė, Ingrida Balnytė, Jolita Palubinskienė, Vaiva Lesauskaitė
Pharmaceutics.2023; 15(12): 2715. CrossRef - Contrasting effects of whole-body and hepatocyte-specific deletion of the RNA polymerase III repressor Maf1 in the mouse
Gilles Willemin, François Mange, Viviane Praz, Séverine Lorrain, Pascal Cousin, Catherine Roger, Ian M. Willis, Nouria Hernandez
Frontiers in Molecular Biosciences.2023;[Epub] CrossRef - Reprogramming of glucose metabolism of cumulus cells and oocytes and its therapeutic significance
Shogo Imanaka, Hiroshi Shigetomi, Hiroshi Kobayashi
Reproductive Sciences.2022; 29(3): 653. CrossRef - Renal denervation ameliorates cardiac metabolic remodeling in diabetic cardiomyopathy rats by suppressing renal SGLT2 expression
Jun-Yu Huo, Wan-Ying Jiang, Shi-Geng Zhang, Yi-Ting Lyu, Jie Geng, Meng Chen, Yuan-Yuan Chen, Zhi-Xin Jiang, Qi-Jun Shan
Laboratory Investigation.2022; 102(4): 341. CrossRef - Alteration in glycolytic/cholesterogenic gene expression is associated with bladder cancer prognosis and immune cell infiltration
Yuying Zhang, Baoyi Zhu, Yi Cai, Sihua Zhu, Hongjun Zhao, Xiaoling Ying, Chonghe Jiang, Jianwen Zeng
BMC Cancer.2022;[Epub] CrossRef - Protein phosphorylation in hemocytes of Fenneropenaeus chinensis in response to white spot syndrome virus infection
Xiaoqian Tang, Ting Liu, Xiaoai Li, Xiuzhen Sheng, Jing Xing, Heng Chi, Wenbin Zhan
Fish & Shellfish Immunology.2022; 122: 106. CrossRef - The Critical Role Played by Mitochondrial MITF Serine 73 Phosphorylation in Immunologically Activated Mast Cells
Lakshmi Bhargavi Paruchuru, Sharmila Govindaraj, Ehud Razin
Cells.2022; 11(3): 589. CrossRef - Glycemic Control and the Heart: The Tale of Diabetic Cardiomyopathy Continues
Miriam Longo, Lorenzo Scappaticcio, Paolo Cirillo, Antonietta Maio, Raffaela Carotenuto, Maria Ida Maiorino, Giuseppe Bellastella, Katherine Esposito
Biomolecules.2022; 12(2): 272. CrossRef - Metabolic Reprogramming in Response to Alterations of Mitochondrial DNA and Mitochondrial Dysfunction in Gastric Adenocarcinoma
Tzu-Ching Chang, Hui-Ting Lee, Siao-Cian Pan, Shih-Han Cho, Chieh Cheng, Liang-Hung Ou, Chia-I Lin, Chen-Sung Lin, Yau-Huei Wei
International Journal of Molecular Sciences.2022; 23(3): 1857. CrossRef - Toxicological effects of tris(1,3-dichloro-2-propyl) phosphate in oyster Crassostrea gigas using proteomic and phosphoproteomic analyses
Chengcheng Yin, Zuodeng Sun, Chenglong Ji, Fei Li, Huifeng Wu
Journal of Hazardous Materials.2022; 434: 128824. CrossRef - Water-Extracted Prunella vulgaris Alleviates Endometriosis by Reducing Aerobic Glycolysis
Min Kyoung Cho, Ling Jin, Jung Ho Han, Jung-Suk Jin, Se-Yun Cheon, Su Shin, Sung-Jin Bae, Jang-Kyung Park, Ki-Tae Ha
Frontiers in Pharmacology.2022;[Epub] CrossRef - Pyruvate Dehydrogenase A1 Phosphorylated by Insulin Associates with Pyruvate Kinase M2 and Induces LINC00273 through Histone Acetylation
Abu Jubayer Hossain, Rokibul Islam, Jae-Gyu Kim, Oyungerel Dogsom, Kim Cuong Cap, Jae-Bong Park
Biomedicines.2022; 10(6): 1256. CrossRef - Celastrol alleviates high-fat diet-induced obesity via enhanced muscle glucose utilization and mitochondrial oxidative metabolism-mediated upregulation of pyruvate dehydrogenase complex
Mohamad Hafizi Abu Bakar, Nor Shafiqah Nor Shahril, Mohamad Shamil Faris Mohamad Khalid, Sharifah Mohammad, Khairul Anuar Shariff, Thiruventhan Karunakaran, Rabeta Mohd Salleh, Mohamad Norisham Mohamad Rosdi
Toxicology and Applied Pharmacology.2022; 449: 116099. CrossRef - Regulation of Metabolism by Mitochondrial MUL1 E3 Ubiquitin Ligase
Lucia Cilenti, Rohit Mahar, Jacopo Di Gregorio, Camilla T. Ambivero, Matthew E. Merritt, Antonis S. Zervos
Frontiers in Cell and Developmental Biology.2022;[Epub] CrossRef - The Pyruvate Dehydrogenase Complex Mitigates LPS-Induced Endothelial Barrier Dysfunction by Metabolic Regulation
Liangfeng Mao, Maomao Sun, Zhenfeng Chen, Zhenhua Zeng, Jie Wu, Zhongqing Chen, Weijin Zhang, Qiaobing Huang
Shock.2022; 57(6): 308. CrossRef - Review: Influence of postabsorptive metabolism on essential amino acid partitioning in lactating dairy cows
J.P. Cant, G.C. Reyes, D.J. Seymour
animal.2022; 16: 100573. CrossRef - T Cells Mediate Kidney Tubular Injury via Impaired PDHA1 and Autophagy in Type 1 Diabetes
Chung-Hsing Wang, Wen-Li Lu, Shang-Lun Chiang, Tsung-Hsun Tsai, Su-Ching Liu, Chia-Hung Hsieh, Pen-Hua Su, Chih-Yang Huang, Fuu-Jen Tsai, Yu-Jung Lin, Yu-Nan Huang
The Journal of Clinical Endocrinology & Metabolism.2022; 107(9): 2556. CrossRef - Pyruvate Dehydrogenase Kinase Protects Dopaminergic Neurons from Oxidative Stress in Drosophila DJ-1 Null Mutants
Yoonjeong Lee, Jaehyeon Kim, Hyunjin Kim, Ji Eun Han, Sohee Kim, Kyong-hwa Kang, Donghoon Kim, Jong-Min Kim, Hyongjong Koh
Molecules and Cells.2022; 45(7): 454. CrossRef - Metabolic regulation of T cell development
Mengdi Zhang, Xiaoxi Lin, Zhou Yang, Xia Li, Zhiguang Zhou, Paul E. Love, Jiaqi Huang, Bin Zhao
Frontiers in Immunology.2022;[Epub] CrossRef - Noncanonical PDK4 action alters mitochondrial dynamics to affect the cellular respiratory status
Themis Thoudam, Dipanjan Chanda, Ibotombi Singh Sinam, Byung-Gyu Kim, Mi-Jin Kim, Chang Joo Oh, Jung Yi Lee, Min-Ji Kim, Soo Yeun Park, Shin Yup Lee, Min-Kyo Jung, Ji Young Mun, Robert A. Harris, Naotada Ishihara, Jae-Han Jeon, In-Kyu Lee
Proceedings of the National Academy of Sciences.2022;[Epub] CrossRef - Frontiers in the enzymology of thiamin diphosphate-dependent enzymes
Sabin Prajapati, Fabian Rabe von Pappenheim, Kai Tittmann
Current Opinion in Structural Biology.2022; 76: 102441. CrossRef - The Role of the Pyruvate Dehydrogenase Complex in the Development of Ischemic-Reperfusion Syndrome
K. A. Popov, Ya. E. Denisova, I. M. Bykov, I. Yu. Tsymbalyuk, G. A. Ermakova, A. G. Zavgorodnyaya, A. S. Shevchenko
Kuban Scientific Medical Bulletin.2022; 29(4): 75. CrossRef - The fragility of liver glycogen from humans with type 2 diabetes: A pilot study
Ziyi Wang, Xiaobo Min, Zhenxia Hu, Mitchell A. Sullivan, Yong Tang, Liang Wang, Robert G. Gilbert, Chen Shi, Bin Deng
International Journal of Biological Macromolecules.2022; 221: 83. CrossRef - Distinct role of mitochondrial function and protein kinase C in intimal and medial calcification in vitro
Marina A. Heuschkel, Anne Babler, Jonas Heyn, Emiel P. C. van der Vorst, Marja Steenman, Maren Gesper, Ben A. Kappel, David Magne, Yann Gouëffic, Rafael Kramann, Willi Jahnen-Dechent, Nikolaus Marx, Thibaut Quillard, Claudia Goettsch
Frontiers in Cardiovascular Medicine.2022;[Epub] CrossRef - Metabolomic responses in livers of female and male zebrafish (Danio rerio) following prolonged exposure to environmental levels of zinc oxide nanoparticles
Xiaohong Wang, Siying Chen, Yingju Qin, Haiqing Wang, Zhenda Liang, Yuanhui Zhao, Li Zhou, Christopher J. Martyniuk
Aquatic Toxicology.2022; 253: 106333. CrossRef - A Missense Variant in PDK1 Associated with Severe Neurodevelopmental Delay and Epilepsy
Raquel Vaz, Josephine Wincent, Najla Elfissi, Kristina Rosengren Forsblad, Maria Pettersson, Karin Naess, Anna Wedell, Anna Wredenberg, Anna Lindstrand, Sofia Ygberg
Biomedicines.2022; 10(12): 3171. CrossRef - Rg3 regulates myocardial pyruvate metabolism via P300-mediated dihydrolipoamide dehydrogenase 2-hydroxyisobutyrylation in TAC-induced cardiac hypertrophy
Jingyu Ni, Hao Zhang, Xiaodan Wang, Zhihao Liu, Tong Nie, Lan Li, Jing Su, Yan Zhu, Chuanrui Ma, Yuting Huang, Jingyuan Mao, Xiumei Gao, Guanwei Fan
Cell Death & Disease.2022;[Epub] CrossRef - Glutamine mitigates murine burn sepsis by supporting macrophage M2 polarization through repressing the SIRT5-mediated desuccinylation of pyruvate dehydrogenase
Yuanfeng Zhu, Xiaoli Chen, Yongling Lu, Lin Xia, Shijun Fan, Qianying Huang, Xin Liu, Xi Peng
Burns & Trauma.2022;[Epub] CrossRef - Loss of metabolic flexibility as a result of overexpression of pyruvate dehydrogenase kinases in muscle, liver and the immune system: Therapeutic targets in metabolic diseases
Jae‐Han Jeon, Themis Thoudam, Eun Jung Choi, Min‐Ji Kim, Robert A Harris, In‐Kyu Lee
Journal of Diabetes Investigation.2021; 12(1): 21. CrossRef - Insulin resistance is mechanistically linked to hepatic mitochondrial remodeling in non-alcoholic fatty liver disease
Chris E. Shannon, Mukundan Ragavan, Juan Pablo Palavicini, Marcel Fourcaudot, Terry M Bakewell, Ivan A. Valdez, Iriscilla Ayala, Eunsook S. Jin, Muniswamy Madesh, Xianlin Han, Matthew E. Merritt, Luke Norton
Molecular Metabolism.2021; 45: 101154. CrossRef - Anti-Warburg Effect of Melatonin: A Proposed Mechanism to Explain its Inhibition of Multiple Diseases
Russel J. Reiter, Ramaswamy Sharma, Sergio Rosales-Corral
International Journal of Molecular Sciences.2021; 22(2): 764. CrossRef - Hyperpolarized magnetic resonance shows that the anti‐ischemic drug meldonium leads to increased flux through pyruvate dehydrogenase in vivo resulting in improved post‐ischemic function in the diabetic heart
Dragana Savic, Vicky Ball, Lorenz Holzner, David Hauton, Kerstin N. Timm, M. Kate Curtis, Lisa C. Heather, Damian J. Tyler
NMR in Biomedicine.2021;[Epub] CrossRef - Beyond the Warburg Effect: Oxidative and Glycolytic Phenotypes Coexist within the Metabolic Heterogeneity of Glioblastoma
Tomás Duraj, Noemí García-Romero, Josefa Carrión-Navarro, Rodrigo Madurga, Ana Ortiz de Mendivil, Ricardo Prat-Acin, Lina Garcia-Cañamaque, Angel Ayuso-Sacido
Cells.2021; 10(2): 202. CrossRef - Detailed evaluation of pyruvate dehydrogenase complex inhibition in simulated exercise conditions
Bodhi A. Jelinek, Michael A. Moxley
Biophysical Journal.2021; 120(5): 936. CrossRef - Proteomics Approach of Rapamycin Anti-Tumoral Effect on Primary and Metastatic Canine Mammary Tumor Cells In Vitro
Patrícia F. Lainetti, Antonio F. Leis-Filho, Priscila E. Kobayashi, Laíza S. de Camargo, Renee Laufer-Amorim, Carlos E. Fonseca-Alves, Fabiana F. Souza
Molecules.2021; 26(5): 1213. CrossRef - Remodeling of Cancer-Specific Metabolism under Hypoxia with Lactate Calcium Salt in Human Colorectal Cancer Cells
Keun-Yeong Jeong, Jae-Jun Sim, Min Hee Park, Hwan Mook Kim
Cancers.2021; 13(7): 1518. CrossRef - The Metabolic Fates of Pyruvate in Normal and Neoplastic Cells
Edward V. Prochownik, Huabo Wang
Cells.2021; 10(4): 762. CrossRef - The Multifaceted Roles of Zinc in Neuronal Mitochondrial Dysfunction
Hilary Y. Liu, Jenna R. Gale, Ian J. Reynolds, John H. Weiss, Elias Aizenman
Biomedicines.2021; 9(5): 489. CrossRef - Melatonin inhibits lung cancer development by reversing the Warburg effect via stimulating the SIRT3/PDH axis
Xiangyun Chen, Bingjie Hao, Dan Li, Russel J. Reiter, Yidong Bai, Baigenzhin Abay, Guojie Chen, Shumeng Lin, Tiansheng Zheng, Yanbei Ren, Xiao Xu, Ming Li, Lihong Fan
Journal of Pineal Research.2021;[Epub] CrossRef - Differential expression of pyruvate dehydrogenase E1A and its inactive phosphorylated form among breast cancer subtypes
Dana M. Zaher, Iman M. Talaat, Amal Hussein, Mahmood Y. Hachim, Hany A. Omar
Life Sciences.2021; 284: 119885. CrossRef - Structure of the native pyruvate dehydrogenase complex reveals the mechanism of substrate insertion
Jana Škerlová, Jens Berndtsson, Hendrik Nolte, Martin Ott, Pål Stenmark
Nature Communications.2021;[Epub] CrossRef - Melatonin: Regulation of Biomolecular Condensates in Neurodegenerative Disorders
Doris Loh, Russel J. Reiter
Antioxidants.2021; 10(9): 1483. CrossRef - Comprehensive Interrogation of Metabolic and Bioenergetic Responses of Early-Staged Zebrafish (Danio rerio) to a Commercial Copper Hydroxide Nanopesticide
Xiaohong Wang, Yingju Qin, Xiaoyu Li, Bing Yan, Christopher J. Martyniuk
Environmental Science & Technology.2021;[Epub] CrossRef - Conditional Knockout of Pdha1 in Mouse Hippocampus Impairs Cognitive Function: The Possible Involvement of Lactate
Wanxin Chen, Xiaoxia Sun, Libin Zhan, Wen Zhou, Tingting Bi
Frontiers in Neuroscience.2021;[Epub] CrossRef - Dihydrolipoamide dehydrogenase, pyruvate oxidation, and acetylation-dependent mechanisms intersecting drug iatrogenesis
I. F. Duarte, J. Caio, M. F. Moedas, L. A. Rodrigues, A. P. Leandro, I. A. Rivera, M. F. B. Silva
Cellular and Molecular Life Sciences.2021; 78(23): 7451. CrossRef - Advanced Bioinformatics Tools in the Pharmacokinetic Profiles of Natural and Synthetic Compounds with Anti-Diabetic Activity
Ana Maria Udrea, Gratiela Gradisteanu Pircalabioru, Anca Andreea Boboc, Catalina Mares, Andra Dinache, Maria Mernea, Speranta Avram
Biomolecules.2021; 11(11): 1692. CrossRef - Obesidade e infecção por SARS-CoV-2: papel da metainflamação
Ana Luísa Silva Albertoni, Luis Gustavo Silva Albertoni, Patricia Elaine de Almeida
HU Revista.2021; 46: 1. CrossRef - Influenza and obesity: its odd relationship and the lessons for COVID-19 pandemic
Livio Luzi, Maria Grazia Radaelli
Acta Diabetologica.2020; 57(6): 759. CrossRef - Acquisition of exogenous fatty acids renders apicoplast-based biosynthesis dispensable in tachyzoites of Toxoplasma
Xiaohan Liang, Jianmin Cui, Xuke Yang, Ningbo Xia, Yaqiong Li, Junlong Zhao, Nishith Gupta, Bang Shen
Journal of Biological Chemistry.2020; 295(22): 7743. CrossRef - Stimulation of glycolysis promotes cardiomyocyte proliferation after injury in adult zebrafish
Ryuichi Fukuda, Rubén Marín‐Juez, Hadil El‐Sammak, Arica Beisaw, Radhan Ramadass, Carsten Kuenne, Stefan Guenther, Anne Konzer, Aditya M Bhagwat, Johannes Graumann, Didier YR Stainier
EMBO reports.2020;[Epub] CrossRef - Fat mass affects nutritional status of ICU COVID-19 patients
Antonino De Lorenzo, Maria Grazia Tarsitano, Carmela Falcone, Laura Di Renzo, Lorenzo Romano, Sebastiano Macheda, Anna Ferrarelli, Demetrio Labate, Marco Tescione, Federico Bilotta, Paola Gualtieri
Journal of Translational Medicine.2020;[Epub] CrossRef - Conditional knockout of pyruvate dehydrogenase in mouse pancreatic β‑cells causes morphological and functional changes
Xiao Wang, Shuchang Lai, Yanshi Ye, Yuanyuan Hu, Daoyan Pan, Xiaochun Bai, Jie Shen
Molecular Medicine Reports.2020;[Epub] CrossRef - Beneficial Effects of Acetyl-DL-Leucine (ADLL) in a Mouse Model of Sandhoff Disease
Ecem Kaya, David A. Smith, Claire Smith, Barry Boland, Michael Strupp, Frances M. Platt
Journal of Clinical Medicine.2020; 9(4): 1050. CrossRef - Fetal circulating human resistin increases in diabetes during pregnancy and impairs placental mitochondrial biogenesis
Shaoning Jiang, April M. Teague, Jeanie B. Tryggestad, Timothy J. Lyons, Steven D. Chernausek
Molecular Medicine.2020;[Epub] CrossRef - SARS-CoV-2-host dynamics: Increased risk of adverse outcomes of COVID-19 in obesity
Rakhee Yadav, Sandeep Aggarwal, Archna Singh
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2020; 14(5): 1355. CrossRef - Effect of flaxseed oil on muscle protein loss and carbohydrate oxidation impairment in a pig model after lipopolysaccharide challenge
Ping Kang, Yang Wang, Xiangen Li, Zhicheng Wan, Xiuying Wang, Huiling Zhu, Chunwei Wang, Shengjun Zhao, Huifu Chen, Yulan Liu
British Journal of Nutrition.2020; 123(8): 859. CrossRef - Arrangement and symmetry of the fungal E3BP-containing core of the pyruvate dehydrogenase complex
B. O. Forsberg, S. Aibara, R. J. Howard, N. Mortezaei, E. Lindahl
Nature Communications.2020;[Epub] CrossRef - The potential impacts of obesity on COVID-19
Ahmed Abdalazim Dafallah Albashir
Clinical Medicine.2020; 20(4): e109. CrossRef - Selective dietary polyphenols induce differentiation of human osteoblasts by adiponectin receptor 1-mediated reprogramming of mitochondrial energy metabolism
Subhashis Pal, Konica Porwal, Sangam Rajak, Rohit A. Sinha, Naibedya Chattopadhyay
Biomedicine & Pharmacotherapy.2020; 127: 110207. CrossRef - Factors Associated with Increased Morbidity and Mortality of Obese and Overweight COVID-19 Patients
Amany Magdy Beshbishy, Helal F. Hetta, Diaa E. Hussein, Abdullah A. Saati, Christian C. Uba, Nallely Rivero-Perez, Adrian Zaragoza-Bastida, Muhammad Ajmal Shah, Tapan Behl, Gaber El-Saber Batiha
Biology.2020; 9(9): 280. CrossRef - Increased Glucose Availability Attenuates Myocardial Ketone Body Utilization
Manoja K. Brahma, Chae‐Myeong Ha, Mark E. Pepin, Sobuj Mia, Zhihuan Sun, John C. Chatham, Kirk M. Habegger, Evan Dale Abel, Andrew J. Paterson, Martin E. Young, Adam R. Wende
Journal of the American Heart Association.2020;[Epub] CrossRef - Stimulating pyruvate dehydrogenase complex reduces itaconate levels and enhances TCA cycle anabolic bioenergetics in acutely inflamed monocytes
Xuewei Zhu, David Long, Manal Zabalawi, Brian Ingram, Barbara K. Yoza, Peter W. Stacpoole, Charles E. McCall
Journal of Leukocyte Biology.2020; 107(3): 467. CrossRef - Short-term high-fat diet intake leads to exacerbation of concanavalin A-induced liver injury through the induction of procoagulation state
Eri Nanizawa, Yuki Tamaki, Reika Sono, Rintaro Miyashita, Yumi Hayashi, Ayumu Kanbe, Hiroyasu Ito, Tetsuya Ishikawa
Biochemistry and Biophysics Reports.2020; 22: 100736. CrossRef - Role of the Mitochondrial Pyruvate Carrier in the Occurrence of Metabolic Inflexibility in Drosophila melanogaster Exposed to Dietary Sucrose
Chloé J. Simard, Mohamed Touaibia, Eric Pierre Allain, Etienne Hebert-Chatelain, Nicolas Pichaud
Metabolites.2020; 10(10): 411. CrossRef - Metabolic shift underlies recovery in reversible infantile respiratory chain deficiency
Denisa Hathazi, Helen Griffin, Matthew J Jennings, Michele Giunta, Christopher Powell, Sarah F Pearce, Benjamin Munro, Wei Wei, Veronika Boczonadi, Joanna Poulton, Angela Pyle, Claudia Calabrese, Aurora Gomez‐Duran, Ulrike Schara, Robert D S Pitceathly, M
The EMBO Journal.2020;[Epub] CrossRef - Increased pyruvate dehydrogenase activity in skeletal muscle of growth-restricted ovine fetuses
Alexander L. Pendleton, Laurel R. Humphreys, Melissa A. Davis, Leticia E. Camacho, Miranda J. Anderson, Sean W. Limesand
American Journal of Physiology-Regulatory, Integrative and Comparative Physiology.2019; 317(4): R513. CrossRef - Recent advances in the pathogenesis of microvascular complications in diabetes
Sungmi Park, Hyeon-Ji Kang, Jae-Han Jeon, Min-Ji Kim, In-Kyu Lee
Archives of Pharmacal Research.2019; 42(3): 252. CrossRef - Secondary Metabolites of The Endophytic Fungus Alternaria alternata JS0515 Isolated from Vitex rotundifolia and Their Effects on Pyruvate Dehydrogenase activity
Changyeol Lee, Wei Li, Sunghee Bang, Sun Joo Lee, Nam-young Kang, Soonok Kim, Tae In Kim, Younghoon Go, Sang Hee Shim
Molecules.2019; 24(24): 4450. CrossRef - Adaptations in Protein Expression and Regulated Activity of Pyruvate Dehydrogenase Multienzyme Complex in Human Systolic Heart Failure
Freya L. Sheeran, Julie Angerosa, Norman Y. Liaw, Michael M. Cheung, Salvatore Pepe
Oxidative Medicine and Cellular Longevity.2019; 2019: 1. CrossRef - Progression of Multifaceted Immune Cells in Atherosclerotic Development
Sungmi Park, In-Kyu Lee
Journal of Lipid and Atherosclerosis.2019; 8(1): 15. CrossRef - SIRT3 elicited an anti‐Warburg effect through HIF1α/PDK1/PDHA1 to inhibit cholangiocarcinoma tumorigenesis
Lei Xu, Yang Li, Lixing Zhou, Robert Gregory Dorfman, Li Liu, Rui Cai, Chenfei Jiang, Dehua Tang, Yuming Wang, Xiaoping Zou, Lei Wang, Mingming Zhang
Cancer Medicine.2019; 8(5): 2380. CrossRef - Differential Mechanism of ATP Production Occurs in Response to Succinylacetone in Colon Cancer Cells
Lee, Woo, Yoo, Cho, Kim
Molecules.2019; 24(19): 3575. CrossRef - Myocardial Adaptation in Pseudohypoxia: Signaling and Regulation of mPTP via Mitochondrial Connexin 43 and Cardiolipin
Miroslav Ferko, Natália Andelová, Barbara Szeiffová Bačová, Magdaléna Jašová
Cells.2019; 8(11): 1449. CrossRef - NDUFAB1 protects against obesity and insulin resistance by enhancing mitochondrial metabolism
Rufeng Zhang, Tingting Hou, Heping Cheng, Xianhua Wang
The FASEB Journal.2019; 33(12): 13310. CrossRef - Mitochondrial Flexibility of Breast Cancers: A Growth Advantage and a Therapeutic Opportunity
Angelica Avagliano, Maria Rosaria Ruocco, Federica Aliotta, Immacolata Belviso, Antonello Accurso, Stefania Masone, Stefania Montagnani, Alessandro Arcucci
Cells.2019; 8(5): 401. CrossRef - Effects of intermittent hypoxia training on leukocyte pyruvate dehydrogenase kinase 1 (PDK-1) mRNA expression and blood insulin level in prediabetes patients
Tetiana V. Serebrovska, Alla G. Portnychenko, Vladimir I. Portnichenko, Lei Xi, Egor Egorov, Ivanna Antoniuk-Shcheglova, Svitlana Naskalova, Valeriy B. Shatylo
European Journal of Applied Physiology.2019; 119(3): 813. CrossRef
- Mitochondria dysfunction induced by decyl-TPP mitochondriotropic antioxidant based on caffeic acid AntiOxCIN6 sensitizes cisplatin lung anticancer therapy due to a remodeling of energy metabolism
- Letter: Patient Understanding of Hypoglycemia in Tertiary Referral Centers (
Diabetes Metab J 2018;42:43-52) - Jae-Han Jeon
- Diabetes Metab J. 2018;42(2):173-174. Published online April 19, 2018
- DOI: https://doi.org/10.4093/dmj.2018.42.2.173
- 2,476 View
- 27 Download
- Letter: Efficacy of Moderate Intensity Statins in the Treatment of Dyslipidemia in Korean Patients with Type 2 Diabetes Mellitus (
Diabetes Metab J 2017;41:23-30) - Jae-Han Jeon
- Diabetes Metab J. 2017;41(2):150-151. Published online April 14, 2017
- DOI: https://doi.org/10.4093/dmj.2017.41.2.150
- 2,760 View
- 35 Download
- Complications
- Renoprotective Effect of Gemigliptin, a Dipeptidyl Peptidase-4 Inhibitor, in Streptozotocin-Induced Type 1 Diabetic Mice
- Gwon-Soo Jung, Jae-Han Jeon, Mi Sun Choe, Sung-Woo Kim, In-Kyu Lee, Mi-Kyung Kim, Keun-Gyu Park
- Diabetes Metab J. 2016;40(3):211-221. Published online March 31, 2016
- DOI: https://doi.org/10.4093/dmj.2016.40.3.211
- 6,345 View
- 53 Download
- 22 Web of Science
- 22 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Dipeptidyl peptidase-4 (DPP-4) inhibitors are widely used in the treatment of patients with type 2 diabetes and have proven protective effects on diabetic kidney disease (DKD). Whether DPP-4 inhibitors have renoprotective effects on insulin-deficient type 1 diabetes has not been comprehensively examined. The aim of this study was to determine whether gemigliptin, a new DPP-4 inhibitor, has renoprotective effects in streptozotocin (STZ)-induced type 1 diabetic mice.
Methods Diabetes was induced by intraperitoneal administration of a single dose of STZ. Mice with diabetes were treated without or with gemigliptin (300 mg/kg) for 8 weeks. Morphological changes of the glomerular basement membrane (GBM) were observed by electron microscopy and periodic-acid Schiff staining. In addition, we measured blood glucose and urinary albumin excretion and evaluated fibrotic markers using immunohistochemical staining, quantitative reverse transcription polymerase chain reaction analysis, and Western blot analysis.
Results Gemigliptin did not reduce the blood glucose levels of STZ-treated mice. In gemigliptin-treated mice with STZ, a significant reduction in urinary albumin excretion and GBM thickness was observed. Immunohistological examination revealed that gemigliptin attenuated renal fibrosis induced by STZ and decreased extracellular matrix protein levels, including those of type I collagen and fibronectin, and Smad3 phosphorylation. In cultured rat renal cells, gemigliptin inhibited transforming growth factor β-stimulated type I collagen and fibronectin mRNA and protein levels via down-regulation of Smad3 phosphorylation.
Conclusion Our data demonstrate that gemigliptin has renoprotective effects on DKD, regardless of its glucose-lowering effect, suggesting that it could be used to prevent DKD, including in patients with type 1 diabetes.
-
Citations
Citations to this article as recorded by- Diabetic fibrosis
Izabela Tuleta, Nikolaos G. Frangogiannis
Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease.2021; 1867(4): 166044. CrossRef - Protective roles of thymoquinone and vildagliptin in manganese-induced nephrotoxicity in adult albino rats
Heba El-Sayed Mostafa, Eman Ahmed Alaa El-Din, Dalia Abdallah El-Shafei, Nehal S. Abouhashem, Aisha Abdallah Abouhashem
Environmental Science and Pollution Research.2021; 28(24): 31174. CrossRef - Evogliptin, a Dipeptidyl Peptidase-4 Inhibitor, Attenuates Renal Fibrosis Caused by Unilateral Ureteral Obstruction in Mice
Mi-Jin Kim, Na-young Kim, Yun-A Jung, Seunghyeong Lee, Gwon-Soo Jung, Jung-Guk Kim, In-Kyu Lee, Sungwoo Lee, Yeon-Kyung Choi, Keun-Gyu Park
Diabetes & Metabolism Journal.2020; 44(1): 186. CrossRef - Gemigliptin Attenuates Renal Fibrosis Through Down-Regulation of the NLRP3 Inflammasome
Jung Beom Seo, Yeon-Kyung Choi, Hye-In Woo, Yun-A Jung, Sungwoo Lee, Seunghyeong Lee, Mihyang Park, In-Kyu Lee, Gwon-Soo Jung, Keun-Gyu Park
Diabetes & Metabolism Journal.2019; 43(6): 830. CrossRef - Recent advances in the pathogenesis of microvascular complications in diabetes
Sungmi Park, Hyeon-Ji Kang, Jae-Han Jeon, Min-Ji Kim, In-Kyu Lee
Archives of Pharmacal Research.2019; 42(3): 252. CrossRef - Diabetic nephropathy: An update on pathogenesis and drug development
Vikram Rao A/L B Vasanth Rao, Sean Hong Tan, Mayuren Candasamy, Subrat Kumar Bhattamisra
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2019; 13(1): 754. CrossRef - Mechanisms and pathways of anti‐inflammatory activity of DPP‐4 inhibitors in cardiovascular and renal protection
Katarina Tomovic, Jelena Lazarevic, Gordana Kocic, Marina Deljanin‐Ilic, Marko Anderluh, Andrija Smelcerovic
Medicinal Research Reviews.2019; 39(1): 404. CrossRef - Effects of Dipeptidyl Peptidase-4 Inhibitors on Renal Outcomes in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis
Jae Hyun Bae, Sunhee Kim, Eun-Gee Park, Sin Gon Kim, Seokyung Hahn, Nam Hoon Kim
Endocrinology and Metabolism.2019; 34(1): 80. CrossRef - The emerging role of novel antihyperglycemic agents in the treatment of heart failure and diabetes: A focus on cardiorenal outcomes
Kelly R. McHugh, Adam D. DeVore, Robert J. Mentz, Daniel Edmonston, Jennifer B. Green, Adrian F. Hernandez
Clinical Cardiology.2018; 41(9): 1259. CrossRef - Acute Kidney Injury and Progression of Diabetic Kidney Disease
Samuel Mon-Wei Yu, Joseph V. Bonventre
Advances in Chronic Kidney Disease.2018; 25(2): 166. CrossRef - Recombinant human GLP-1(rhGLP-1) alleviating renal tubulointestitial injury in diabetic STZ-induced rats
Weiqin Yin, Shiqing Xu, Zai Wang, Honglin Liu, Liang Peng, Qing Fang, Tingting Deng, Wenjian Zhang, Jinning Lou
Biochemical and Biophysical Research Communications.2018; 495(1): 793. CrossRef - Chronic inhalation of e-cigarette vapor containing nicotine disrupts airway barrier function and induces systemic inflammation and multiorgan fibrosis in mice
Laura E. Crotty Alexander, Christopher A. Drummond, Mark Hepokoski, Denzil Mathew, Alex Moshensky, Andrew Willeford, Soumita Das, Prabhleen Singh, Zach Yong, Jasmine H. Lee, Kevin Vega, Ashley Du, John Shin, Christian Javier, Jiang Tian, Joan Heller Brown
American Journal of Physiology-Regulatory, Integrative and Comparative Physiology.2018; 314(6): R834. CrossRef - Renoprotective effect of fucoidan from Acaudina molpadioides in streptozotocin/high fat diet-induced type 2 diabetic mice
Shiwei Hu, Jinhui Wang, Jingfeng Wang, Shijie Li, Wei Jiang, Yu Liu
Journal of Functional Foods.2017; 31: 123. CrossRef - Dipeptidyl peptidase-4 inhibition and renoprotection
Yuta Takagaki, Daisuke Koya, Keizo Kanasaki
Current Opinion in Nephrology and Hypertension.2017; 26(1): 56. CrossRef - Treatment of diabetic kidney disease: current and future targets
Mi-Kyung Kim
The Korean Journal of Internal Medicine.2017; 32(4): 622. CrossRef - Pharmacological Treatment in Diabetes Mellitus Type 1 – Insulin and What Else?
Ewa Otto-Buczkowska, Natalia Jainta
International Journal of Endocrinology and Metabolism.2017;[Epub] CrossRef - GLP-1 and the kidney: from physiology to pharmacology and outcomes in diabetes
Marcel H. A. Muskiet, Lennart Tonneijck, Mark M. Smits, Michaël J.B. van Baar, Mark H. H. Kramer, Ewout J. Hoorn, Jaap A. Joles, Daniël H. van Raalte
Nature Reviews Nephrology.2017; 13(10): 605. CrossRef - Efficacy, safety and albuminuria‐reducing effect of gemigliptin in Korean type 2 diabetes patients with moderate to severe renal impairment: A 12‐week, double‐blind randomized study (the GUARD Study)
Sun A. Yoon, Byoung G. Han, Sung G. Kim, Sang Y. Han, Young I. Jo, Kyung H. Jeong, Kook H. Oh, Hyeong C. Park, Sun H. Park, Shin W. Kang, Ki R. Na, Sun W. Kang, Nam H. Kim, Young H. Jang, Seong H. Shin, Dae R. Cha
Diabetes, Obesity and Metabolism.2017; 19(4): 590. CrossRef - Sodium butyrate has context-dependent actions on dipeptidyl peptidase-4 and other metabolic parameters
Eun-Sol Lee, Dong-Sung Lee, Prakash Raj Pandeya, Youn-Chul Kim, Dae-Gil Kang, Ho-Sub Lee, Byung-Chul Oh, Dae Ho Lee
The Korean Journal of Physiology & Pharmacology.2017; 21(5): 519. CrossRef - Lobeglitazone, a Novel Peroxisome Proliferator-Activated Receptor γ Agonist, Attenuates Renal Fibrosis Caused by Unilateral Ureteral Obstruction in Mice
Kwi-Hyun Bae, Jung Beom Seo, Yun-A Jung, Hye-Young Seo, Sun Hee Kang, Hui-Jeon Jeon, Jae Man Lee, Sungwoo Lee, Jung-Guk Kim, In-Kyu Lee, Gwon-Soo Jung, Keun-Gyu Park
Endocrinology and Metabolism.2017; 32(1): 115. CrossRef - Gemigliptin: An Update of Its Clinical Use in the Management of Type 2 Diabetes Mellitus
Sung-Ho Kim, Jung-Hwa Yoo, Woo Je Lee, Cheol-Young Park
Diabetes & Metabolism Journal.2016; 40(5): 339. CrossRef - Risk assessment and management of post-transplant diabetes mellitus
Eugene Han, Myoung Soo Kim, Yu Seun Kim, Eun Seok Kang
Metabolism.2016; 65(10): 1559. CrossRef
- Diabetic fibrosis

 KDA
KDA
 First
First Prev
Prev





